FAILURES OF FRACTIONAL CRYSTALLIZATION
Fractional crystallization is defined as a separation method based on the differences in solubility. It is also a method used in refining substances based on differences in solubility. If a mixture of two or more substances in solution is allowed to crystallize, for example by allowing the temperature of the solution to decrease, the precipitate will contain more of the least soluble substances.
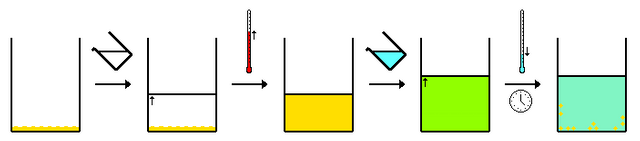
Image Source
The proportion of components in the precipitate will depend on their solubility products. If the solubility products are very similar, a cascade process (this is a process that takes place in a number of steps, usually because the single step is too inefficient to produce the desired result) will be needed to effectuate a complete separation. A solution containing the desired material and associated impurities is cooled or the solvent is allowed to evaporate. Crystals of the desired material, which usually has the highest concentration in the original solution, are normally deposited first, while the impurities remain in solution.
Uses of fractional crystallization
- It is used to separate two or more solid solutes which are present in the same solution in roughly equal amounts.
- It is used in industries where purity of the product is important as in the manufacture of drugs and in sugar production.
- It is used to obtain trace elements in bulk compounds
- It is also used in purification of semiconductors to prepare them for making transistors and integrated circuits.
Successes of fractional crystallization
As a separation method, fractional crystallization is so simple, powerful and inexpensive. It is the method of choice in the chemical industries. It works well for almost everything. Its success stems from the fact that most substances crystallize as pure compounds of a single molecule or a single set of ions.
Failures of fractional crystallization
Failures of Fractional crystallization can best be determined if;
- The impurities have solubility characteristics similar to those of the desired pure component, and both substances consequently co-crystallize.
- The impurity is presence in such large amounts that the crystals meritably become contaminated.
- If a pure substance cannot be produced in a single crystallization.
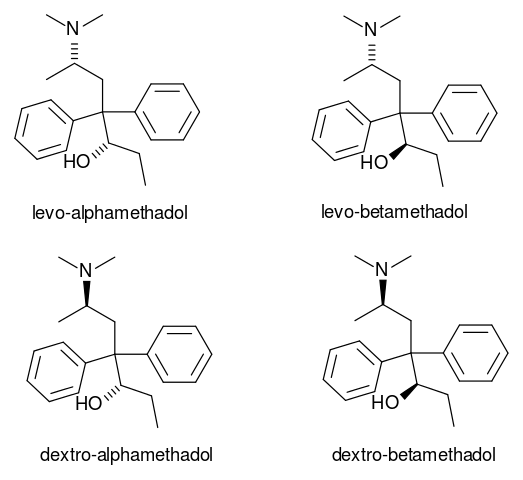
Failures of fractional crystallization are rare. It doesn’t work for pairs of separable (i.e. resolvable) optical isomers (one of two stereoisomers that are mirror images of each other and are not identical). Failures of fractional crystallization imply either the existence of an ordered solid-state compound or of a ‘mixed’ crystal (sometimes called a solid solution). A solid-state compound also known as a co-crystal corresponds to a maximum in a T-X (temperature-composition) phase diagram and has a fixed stoichiometric ratio; such compounds are represented on phase diagram by vertical lines. Mixed crystals, on the other hand, are disordered solids in which several molecules or ions of different types occupy the same site mixed crystals will not be considered further.
Optical Isomers
Solid-state compounds are much more likely to form if there is some strong specific attraction between the molecules. Consider the expected deposition of co-crystals from a solution containing both an acid and a base. A compound is expected because complete proton transfer leads to a salt (such as an ammonium chloride), which can (assuming a favourable arrangement of the ions) have a very low energy relative to crystals of the neutral molecules, and because incomplete proton transfer still gives a compound with hydrogen bonds that are usually better than can be formed by the acid or base alone.
This systemic failure has long been understood as evidence that symmetry operatives of the second kind, especially inversion, are very favourable for crystal packing. In the case of separable optical isomers, the success of fractional crystallization is so surprising that is has been given a special name (i.e. spontaneous resolution).
Interest in the failures of fractional crystallization grew for which there was no obvious reason because some previous authors worked with or knew of several co-crystals formed by isomers.
If the method fails, then the batch may contain disordered mixed crystals (or, solid solutions) or ordered stoichiometric compounds such as solvate. Fractional crystallization is expected to fail for optical isomers, which usually crystallize together to form ordered racemic (i.e. it has equal amounts of left and right handed optical isomers of a chairal molecule) compounds.
A related goal of this post is to identify instances in which fractional crystallization can fail. The existence of quasiracemates is evidence that even an approximate inversion centre is very favourable for crystal packing. The task of making a list of quasiracemates has been characterized as ‘difficult at best and certainly impractical’.
Solution to the failure of fractional crystallization
Solution to the failure of fractional crystallization is partial melting. Partial melting occurs when only a portion of a solid is melted. For mixed substances, such as a rock containing several different minerals or a mineral that can display solid solution, these melt can be different from bulk composition of the solid. It occurs when the solid and liquid temperature are different.
CONCLUSION
In conclusion, fractional crystallization separates two or more substances based on their differences in solubility. The method will fail if the desired material and a co-crystal of it with one of the impurities present have similar solubility. When it fails, it implies either the existence of an ordered solid-state compound or mixed crystals
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
http://www.chem.uky.edu/research/Brock/CoCrys.pdf