The Cool Science of the Slippery Ice
The idea that someone will say ice is slippery is almost the same as someone telling you that fire is hot. Oh yeah, we already know that is how it works as a child. Those who don't, learned the hard way when we stick our pink finger into the hot stove or oven.
The water under particular temperature solidifies and forms ice. The slippery nature of ice makes it perfect for skiing. The reason that ice is slippery is something no one can pinpoint. It is a grey area much the same way we have not gotten to the bottom of what makes a bicycle stable; we pedal it, and it moves, but up to date no scientist/engineer can very much educate us on why it does not keel over and lose stability.

A road sign warns Users of slippery sature when the temperature drops. Image credits: By By abdallahh from Montréal, Canada (Gaspé)CC BY 2.0 from Wikipedia Commons, Link]
The water as it solidifies to ice expands, some school of thought believes that walking on ice decompresses it into water that forms the slippery effect we experience while standing on ice. This decompression by weight on ice's surface is a flawed one. The human mass produces a pressure that is negligible and not enough to cause the formation of water that is enough to reduce the frictional resistance between the feet and the ice surface.
The Irish physicist, John Joly on December 15, 1886, came up with a theory of why ice is slippery. He published a scientific paper for the Scientific proceedings of the Royal Dublin Society which describes the dynamics that made ice-skating possible. He concluded that pressure of the skateboard liquifies the ice forming a thin water film which makes the ice slippery enough for the skaters to glide through.
John was not alone with this perception that they had deciphered the mystery surrounding the slippery ice, a duo P. Bowden and T.P. Huges, in 1939 did some calculation and concluded that the temperature is the culprit. While Dory believed that the pressure exerted by skaters caused a thin layer of water to decrease friction, Bowden and associate arrived at the conclusion that temperature created due to the friction between the skater's footwear and the ice's surface after observing he kinetic and static friction of various ski material such as the wood and metal.
Though scientists such as Samuel Colbeck disagreed on the premise that the melting of ice under pressure or temperature may have anything to do with the ice's slippery nature as skaters enjoy their game at temperatures as low as -35 degree Celsius. The argument was that at such a low temperature, it would be impossible to produce enough pressure or heat to make the ice melt.
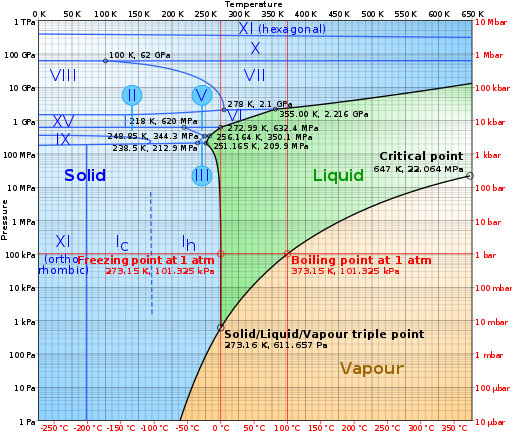
The Water Phase Diagram. Image credits: By CmgleeCC BY-SA 3.0 from Wikipedia Commons, Link]
Looking at the graphical representation of the phases of water, we can identify the seven stages out of the 11 phases of water depicted in the diagram. The temperature at which the ice will melt increases with the rise in pressure with an exception made in the low-pressure range.
Some other theory has it that there is open air trapped on one end of the ice that makes the surface of the ice to maintain liquid water. This theory is one that has been proven to be right. However, like the other theory, the water formed is small and hence does not enough to change the frictional resistance of the feet in contact.
Recently a team of researchers led by two brother Prof Mischa Bonn and Prof Daniel Bonn both from the University of Amsterdam in the Netherlands published their findings on why ice is slippery on the Journal of Physical Chemistry titled Molecular Insight into the Slipperiness of Ice on May 9, 2018.
The team dug deep into understanding the slipperiness of ice by conducting measurements over a wide range of temperature covering - 100-degree Celsius to 0-degree Celsius within speeds of 10-6 to 10-1 meter per second. This range far outstrips the efforts of past researchers who conducted similar findings to understand the typical behaviour of ice which differs from other surfaces.
At the end of their research, the results were in. The team concluded that the result has nothing to do with either temperature which melts the ice or the pressure applied to the surface of the ice. But the findings instead points to the molecular nature of the water and the way that ice melts layer-by-layer.
They observed that the surface of the ice has highly mobile water molecules which facilitates the slipperiness of it when it comes in contact with a ski.
This ice outmost layer is thin and behaves like a liquid at temperatures of up to - 30-degree Celsius. With more precise measuring instrument at their disposal, researchers were able to isolate and observe the molecules that exist on this outer layer.
People often erroneously say that scientists are bored individuals always looking for solutions in things that should be left alone or somewhat "thinking too deep" on mundane issues. But that is not true because the study of ice slipperiness is not something that has no usefulness. First, it comes in handy in road safety, vehicle tire traction on icy roads can be better when we understand what the properties of slippery surfaces.

Tire tracks on a snowy road. Image credits: By ManfredrichterCC0 from Pixabay, Link]
The chemical reactions that occur will further throw more insights into understanding glaciers and the role that ice crystal undertakes in the clouds and its relationship with the ozone layer. Winter sports will benefit immensely from the research also if we can understand more of the complex relationship of the sliding frictions that the ice surface offers over a wide range of conditions.
References
A new study reveals why ice gets so slippery — and it wasn’t what we expected
Twitter Update of News and Annoucement from the National Snow and Ice Data Center (NSIDC)
If you write STEM (Science, Technology, Engineering, and Mathematics) related posts, consider joining #steemSTEM on discord here. If from Nigeria, there may be need to include the #stemng tag in your post. You can visit this blog by @stemng for more details. You can also check this blog post by @steemstem here and this guidelines here for help on how to be a member of @steemstem. Please also check this blog post from @steemstem on proper use of images devoid of copyright issues here
Would you like to delegate to the @steemstem? Here is a link below
50 SP | 100SP | 500SP | 1,000SP | 5,000SP | 10,000SP | 50,000SP

Why ice is slippery has not yet being deciphered.
But in my opinion, I will give a noble prize to the school of thought that professed Ice slips because of pressure exerted on it by someone
Your Nobel prize would have gone to the wrong person. :)
Hahaha. This is so hilarious!
Lol
Thats my opinion though
Yeah :)
:D
Almost everything in this world has the backing of science, it only takes the work of scientist to deanonymize the hidden fact about it.
I've learnt a new thing today, Well written post sir
I just learned a new word: deanonymise :)
Thank you.
Wow! Really, I have never thought about it like this. It's awesome that natural occurrences have scientific undertone.
If I were asked, I would have concluded that it's the pressure exerted by our weight (which would lead to the process of regelation in the ice) that causes ice to be slippery.. and I would've failed it :(
Now I know better. Thanks for sharing
Science keeps bringing us facts over fiction :)
If you can imagine the outcome of a lot of events without science showing us the real state of things; it will be as chaotic as anyone can imagine.
This is a great article. You completely explained the science of skippery nature of ice.
This makes so much sense that it is difficult to doubt it. Science have come a long way, and it feels great to know that every field of scientific interest has real life applications.
I wonder how you were able to get those images to the left and right sides? Will like to know the formatting commands.
Thanks
The markdown may look scary but it is simple once you get to see how it got to be that way. If you change the left to right in the "pull-left" syntax of the markdown, the image will be aligned to the right side of your blog. Here is the markdown here which you can copy and paste and edit the links as appropriate:
<a href="https://commons.wikimedia.org/wiki/File:Phase_diagram_of_water.svg">Link</a></a></sub></center></div>Link in green colour: uploaded image url
Link in red color: the attribution to the original owner of image.
Link encircled by blue color: Image licence
Link encircled by black color: Image Source URL
Great explanation. Thanks for being awesome. Gonna have to try this out!
That will be awesome.
If at all I am to at least choose from the list of the hypothesis given above, I think would have gone for that of John loly.
But a better explanation given at end the end of the article leaves me with no choice but to disregard my initial option.
Brilliant article I must say 🙌
You mean John Joly? He did start off a chain of events which till today is still being worked on.
Coming to what prof mischa bonn and daniel bonn said,i think ice is not dependent on it temperature neither pressure. But its molecular nature and the way it melts.
Yes, that's the finding.
Posted using Partiko Android
If it were just me, I'd say there is no thin layer of water above the ice since water solidification starts from the top. Well maybe I'm wrong, as it may start anywhere and then the ice moves upward due to density differences.
In a different experience, touch the body of a bowl immediately it is taken out of a freezer, and your hand would get stuck for a while, until it melts and then move freely, so I guess the pressure theory is right. If this is correct, I guess friction should be left out. Its either a body solidifies on ice or slips on it. (Still, this may be wrong. Experiments will always bring out the facts)
Well its certain that liquid water causes the slipperiness but its understanding at such low temperatures is the case now.
I don't think any solid is slippery
The people in here did not guess, they actually carried out many measurements to arrive at their conclusion. Things mostly ain't what they seem in the realm of science, it's only proven hypothesis after series of demonstrations that are what can be said to be the real deal.
Posted using Partiko Android
I need to be pedantic here and point out that you're only falsifying hypotheses in good science. Granted, you're often falsifying the null case of the hypothesis you have in mind, and 'failing to reject the null' is a subtle difference from 'proving a hypothesis', but it's important.
As an apology for my pedantry, here's a neat word which is related to your article: tribology.
I like that the falsifying in this instance is in italics. But when carefully examined, the case here is simple, the researchers arrived at the conclusion which was presented as they strive to find a conclusion to that event. In actual sense, people are still working on the project. Should we take their conclusion to be correct? That's a subjective answer which I'd like to hear inputs from the readers.
With the mindset I presented, we should not take their viewpoint to be correct. We can, however, consider it to be less likely to be false than the competing hypothesis.
That, I can totally agree with :D
Posted using Partiko Android
I think i will completely agree with the report of the two prof. It seems really logical owing to the fact that the surface of the ice behave like a liquid more than a solid. Even mere touching the surface of a iced water you will have a soapy or slippry feel. Though i have never given it a second thought before now, but thanks @greenrun for this enlightement
I'm glad you found it useful :)
Posted using Partiko Android
Thank you for your contribution. Dont forget to link references and sources when applicable!
=======================================================================================
This post was upvoted and resteemed by @Steemgridcoin with the aim of promoting discussions surrounding Gridcoin and science.
This service is free. Please follow @steemgridcoin if you want to support this initiative.
Have a nice day. :)
This post has been voted on by the steemstem curation team and voting trail.
There is more to SteemSTEM than just writing posts, check here for some more tips on being a community member. You can also join our discord here to get to know the rest of the community!