Iron as an element; The Biography Of The King Of Metals.
Hello everyone, it's nice to be back again. As always I want to thank you all for the support and encouragement you gave me via your lovely comments, I certainly appreciate it alot. You all are wonderful people.
I saw avengers infinity wars today, honestly I am not a fan of marvel movies (except deadpool), this is the first avengers movie I am seeing. One particular character caught my eye, Iron Man. The way he took hit after hit using his Iron Man suit as shield marvelled me. Although, I'm not sure if the suit was made from iron (I expect it to be else why call him Iron Man), it made me think of iron as an element, its properties, types, usage and even mixing with other elements to form an alloy. I will as usual attempt to walk you through the depths of iron as an element.
Iron itself
What is the most abundant natural occurring metal found in the earth's crust? You are thinking iron right? Well, I am very much delighted to tell you it isn't iron, it is bauxite and co. I meant aluminium by the way. Bauxites are aluminium oxides. Iron actually is the second most abundant naturally occurring metal on earth.
Like aluminium, it isn't found in a natural state (like pure iron) like they way you will find gold. You will always find iron in the company of its friends. Examples of these friends in company with iron include sulphur(in form of pyrites), oxygen (in form of magnetite and haematite ) and carbon with oxygen (in form of siderite ) all the listed friends are what is called the iron ores (which is ironic because Ore in my language means friend). Iron can also be found in the chlorophyll of plants and the pigments of our blood not like we can extract iron from those places though. We don't need iron in company of it's friends, we only need iron so how do we separate iron from its beloved friends.
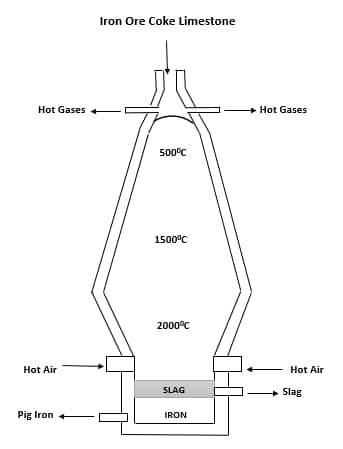
Extraction of Iron
Separating iron from its ore is basically the same for all forms of ore. Let's assume we are picking haematite. Our extraction process will involve extracting iron from haematite. How do we go about this?
First of all, we need a blast furnace which is where the whole extraction takes place, also we need limestone and coke(not Coca-Cola). Haematite is roasted in dry air so iron can change its state from iron (II) to iron (III) ( iron is a transition metal which explains why it has multiple states). The iron (III) produced is then mixed with coke and limestone and poured into a blast furnace wherein it is heated to a very high temperature.
The mixture is poured in to the furnace from the top and at the bottom hot air is blown into the furnace using tuyeres (they are just small pipe that blow hot air). At the top and bottom of the furnace the temperature are different as you can have as high as 2000 degree Celsius at the bottom while the temperature at the top of the furnace might be a very smaller one in 500 degree Celsius.
The coke is forced to change its state (oxidized) by oxygen from the hot air and frees up carbon dioxide in the process. This process is an exothermic one as there are loads of heat released to the surroundings.
As the gas makes it way up the furnace, the carbon dioxide is reduced by coke(again) to carbon monoxide this is due to the absence of oxygen in the upper part of the furnace. The carbon monoxide now reacts with iron (III) oxide (haematite) and reduces it to just iron.
Once the temperature in the furnace gets sufficiently high enough, the limestone breaks down into calcium oxide and carbon dioxide. The calcium oxide immediately reacts with silicon oxide (which is sand) to form calcium trioxosilicate. You may wonder why we need the limestone as the iron we need as already been produced, the limestone is used to remove impurities from the iron formed.
Iron is formed in form of a molten liquid and drops down to the bottom of the furnace where a valve is opened to allow it run into moulds. The impurities (in form of a molten slag) mainly calcium trioxosilicate floats on top of the molten iron and can be easily tapped away.
After the molten iron sets in the mould, the resulting iron is called pig iron, which with little modifications can be turned to cast and wrought iron. This different forms have iron have the unique properties and application.
Forms of iron and their uses.
First off the list is pig iron, are you familiar with the simile that goes thus “as dirty as a pig” I'm very sure this was what those that named this form of iron thought of as they have rightfully named it so. Pig iron is simply put dirty iron, it contains impurities of varying amounts in phosphorus, silicon, manganese and the likes. It also contains about 5% carbon, which is bad as too much carbon along with high impurity content makes it brittle and too hard therefore is unable to resist shear forces hence it has very limited industrial applications. The major use of pig iron is its use in production of cast iron.
Another form of iron is the cast iron, which is just a remix of pig iron. Cast iron is formed by melting pig iron and adding iron scraps, then remelting again as the resulting molten is that of cast iron which can be allowed to set in a mould of one's desired shape. Although, cast iron does not contain as high content of impurities as pig iron, they have fairly the same properties. Since cast iron can't be machined (welded or forged) it finds it application in making of kitchen utensils like stoves and cookers. Also cast iron is used in making lamp posts, railings, etc. All of these items have low tensile strength.
Finally we have the wrought iron, if there were an iron kingdom sons of wrought iron will be the kings as they are the most noble and purest of all the types of iron. They contain only about 0.1% of carbon. This form of iron is gotten by refining cast iron. To make wrought iron, you go back to the beginning, instead of roasting haematite in air to give iron (III) oxide, haematite is mixed with cast iron and burnt in a furnace where all impurities are either removed as gaseous oxide or as slag from the semi-molten mass of iron. After which you have your wrought iron, the only reason why it is better than other forms of carbon is because it has less impurities. Wrought iron industrial usage varies from using in production of nails, chains, horseshoes to making of the cores of an electromagnet. All of these are possible because it is soft(although tough) and malleable.
Have you ever been to cold stone creamery or just any other creamery where you are allowed to design your own ice-cream instead of having one flavor, you can mix vanilla with chocolate, strawberry with vanilla, you could even mix the three flavors together, if you have then you will have no problem understanding what an alloy mean, just replace the ice-creams with metals and you are good. If you haven't been there, an alloy is gotten by adding different types of elements to a base metal to have a desired result.
Alloys of Iron
The major alloy of iron is steel, which is just iron + carbon + any other element based on what the steel is to be used for. The majority of its (steel) properties depends on various factors which includes its carbon content, element which its alloyed with, the heat treatment it receives (mostly tempering) I was about to start discussing about heat treatments, but @rharphelle did justice to that a few weeks ago, educated yourself here
Like iron, steel has various forms too. We have the mild steel which contains carbon content of about 0.1% - 0.25% making it ductile (able to be drawn into a wire), malleable (able to be beaten into sheets) and soft. Mild steels are beloved in the engineering world as many of its uses finds engineering application like it is used in making of car body parts and screws, it's also used in producing steel pipes which are used to transport air or liquids.
Medium steels contains about 0.25 - 0.6% of carbon, with the increased carbon content they are harder than mild steel therefore they are used in making machine tools. With appropriate heat treatment medium steels can be converted into steels of varying degrees of hardness. When the medium steel is heated, it is cooled rapidly and the resulting steel is hard and brittle. In order to remove the brittleness of the steel, it is heated again and allowed to cool naturally. This not only removes the brittleness, it also increases the tensile strength of the steel. If you read @rharphelle’s post already, you will know the process I just described is called tempering. Which makes the resulting steel been called tempered steel, a common example of where this type of steel finds its application is in razor blades.
With iron and carbon only present in steel, other elements can be added to steel, with each element providing it with a unique properties and applications. The most common one might be Stainless Steel which is gotten by adding nickel and chromium. Stainless Steel are very important as they have wide range of application due to their ability to resist corrosion. When manganese is added to steel, the resulting steel is very tough that it is used in making tools for rock drilling. Spring steels are gotten by adding silicon, permanent magnets are made from steels which have cobalt in it.
Conclusion
When classifying metals,we classify them into ferrous and nonferrous metals. With the ferrous metals being the ones with iron in them. This shows clearly that iron is most important element of the metal group. To say iron is the king of all metals might be an understatement, I will say it's the god of metal.
I'm sure by now you know why the Iron Man suit is able to withstand all those hits of great magnitude and Tony Stark (Iron Man) doesn't feel the impact inside the suit.
One knock on iron is its affinity to react with oxygen and moisture, when this happens corrosion is what it is called. Corrosion can cause great destruction, for example if the support of a building corrodes and the building collapses not only will lives be lost, properties will be lost also. Although to curb corrosion, corrosion inhibitors are commonly used these days to either slow down corrosion or even stop corrosion itself.
Thank you all for taking your time to read about Iron as an element.
References
Callister, W. D. and Rethwisch, D. G. Materials science and Engineering - An introduction (Eighth Edition)



The iron comes in almost an infinite number of alloys, pretty much it is everywhere. I should go and watch that movie as well.
It's a good watch, thank you for stopping by. It's nice to have you in my comments.
Ore(friends) that's interesting really.. (lol). Iron is one of my best friend when it comes to metal. It is very important element in engineering metallurgy. Well what else can i say you have succeeded in breaking the bones of iron structure forms and its extraction yourself..well done
Thanks for the lovely comment
Wow, Iron have been a very important raw material in manufacturing engineering. I'm glad to see somebody write on it.
Thanks for stopping by
Iron is such a wonderful element God has blessed us with..it's found very useful in many areas.
Beautiful writeup @addempsea
Thanks man.
I liked it, but you didn't mention Clint Eastwood, "go for the heart," I think it was in the "For a few Dollars More" movie where he had a piece of iron for a breast plate under his poncho when he went up against the bad guy rifleman. Good movie, good post. I like post that bring random memories to the forebrain.
I'm glad you liked it, thanks for dropping by.
Iron is an essential element.... The problem we have is that so many people call anything metal iron.
Yeah, they lack the proper education on what is what. Thanks for the additional information.
Lemouth actually made me know the real name and cast name of "Iron man" some days back
Iron is definitely one of the most common metal around and it forms a major part of the machines we see around..
Nice work you did here with the whole break down and humorous lines. Keep it up
Thanks bro, really appreciate the compliment.
It's a pleasure always
Interesting article spice with humour as usual. I particularly enjoyed the break down
Thanks
Hahaha good intro and related comparisons. I thought I could have a movie review. Interesting post even honestly I don’t get a lot of them :)
It's nice to have you here. Thanks for your comment.
You know this is a great one?!
Post like this and other engneering posts gives me a nod at owning a manufacturing company. The dream has just started anyway 💪
Thanks @addempsea for this.
I believe in your dream.
Thanks for the nice words.