Tramadol abuse
The commercially available product contains the r
Tramadol was first synthesized in 1962 by Grünenthal GmbH in Germany by coupling of the corresponding cyclohexanon with 3-methoxyphenylmagnesium bromide in a Grignard reaction (Grünenthal, 1965; Grünenthal, 1967). More recently, the chemical synthesis of tramadol and two of its metabolites has been described by the same coupling reaction using organolithium derivatives (Alvarado et al., 2005).
Tramadol shows structural resemblance with codeine. Both tramadol and codeine have a 3-methoxy group on the phenyl ring and share O-demethylation than the parent compound. In addition, the dimethyl aminomethyl moiety of tramadol resembles the methylated ring nitrogen of morphine and codeine, and forms an essential part of the pharmacophore that interacts with the µ-opioid receptor and monoamine transporters (Houmes et al., 2004). N-demethylation yields metabolites that lack significant analgesic activity. Tramadol hydrochloride is readily
Drug addiction, or as it is also called, drug dependence, is a serious health problem; in addition to the huge direct health costs (psychiatric and physical), there are massive costs in terms of crime, loss of earnings and productivity, and social damage (David et al., 2006). The processes of addiction involve alterations in brain functions because abused drugs are neuroactive substances that alter brain transmitter function. Drugs of misuse were traditionally classified according to their physiological or psychological actions (eg, stimulants, sedatives). Generally, the more efficacious a drug is at producing its pharmacological effect, the greater is the addiction potential and street value. Drugs with lower efficacy are called partial agonists (buprenorphine for opioid receptors (Lewis et al., 2002), bretazenil for benzodiazepine receptors (Busto et al., 1999). The pharmacological profile of partial agonists is such that they are useful in maintenance treatment since they provide some reinforcement; thus, buprenorphine will keep opioid addicts in treatment. Nonetheless, because partial agonists attenuate the actions of full agonists, buprenorphine should diminish intravenous street heroin use. Moreover, the lower efficacy means that it is much safer in overdose. Antagonists have zero efficacy (eg, naltrexone for opioid receptors, flumazenil for benzodiazepine receptors). They are very effective blockers of agonists. Their limitations are that they can precipitate withdrawal in physically dependent addicts and because they do not provide any reinforcement, there is little incentive for addicts to stay on them. Pharmacokinetic factors are also important in determining the misuse potential of drugs; in general, the faster the drug enters the brain the more reinforcing it is. Addiction to alcohol or drugs of abuse is a chronic disease that has a major impact on well-being for affected individuals and is a great burden for the society. Compulsion to seek and take the drug, loss of control in limiting intake, continued use despite obvious harm, and emergence of a negative emotional states (e.g. anxiety) when the drug is withdrawn are criteria for a diagnose of a substance dependence. However, severe problems in life are often seen long before these criteria are fully met (O'Brien and Gardner, 2005; Volkow and Li, 2005). Several neurobiological theories of addiction have recognized a key role for some component of reward or reinforcement in the development of substance dependence, and often mesolimbic dopamine system is mentioned in this context (Koob, 2006). Specifically, a projection from dopaminergic neurons in the ventral tegmental area to the nucleus accumbens has accumulated interest in biomedical research of addiction. Drugs of abuse share an ability to intervene with this system by altering the function of the dopaminergic cells directly or indirectly through other, e.g. glutamatergic and GABAergic, neurotransmitter systems. Furthermore, repeated exposure to drugs has long-lasting effects within these neural systems increasing the probability to relapse (Robinson and Berridge, 2000). Endogenous opioid system is another important mediator of drug effects that has been implicated in the development and maintenance of addiction. For obvious reasons, it has a significant role in the addictive properties of opiates, but also the rewarding effects of ethanol are thought to be associated with it (Oswald and Wand, 2004). When drugs of abuse are given repeatedly they induce behavioral plasticity that is underlain by neuronal adaptations within the brain areas (e.g. mesolimbic dopamine system) controlling motivational effects of both drugs and natural reinforcers like food and sex (Vanderschuren and Kalivas, 2000; Robinson and Berridge, 2001). Behavioral sensitization is a form of neuronal plasticity where repeated administration of drugs induces a progressive and enduring enhancement in their behavioral and neurochemical effects. Therefore, it can be used as a tool for studying the importance of these phenomena in addiction. Drug addiction is a maladaptive pattern of substance use that leads to clinically significant impairment or distress. Genetical background, environment, stress, and conditioning effects contribute to the vulnerability to enter the cycle that leads from social drug-taking and acute reinforcement to compulsive use and substance dependence. The initiation of drug abuse may be more associated with social and environmental factors, whereas the change to addiction probably is underlain by neurobiological factors. There are several neurobiological theories of addiction, and while they have an emphasis on different aspects of drug addiction, many of these have implicated a key role for some component of reward or reinforcement that is usually mediated through mesolimbic dopamine system (Wise, 1996; Robinson and Berridge, 2000; Weiss and Porrino, 2002; Koob, 2006). Opiates, including morphine, are potent analgesic drugs that can often provide an optimal solution for pain relief. However, their addictive potential is a serious limitation of their use. The rewarding properties of morphine are believed to be primarily mediated via binding to l opioid receptors located on inhibitory interneurons in the ventral tegmental area (Johnson and North, 1992; Jalabert et al., 2011). This signal causes disinhibition of dopaminergic neurons activity and increases dopamine release in the dorsal and ventral striatum, brain regions that are crucial for the development of addiction, further leading to rearrangement of synaptic connections. Inducing structural plasticity of the neuronal network requires alterations of the transcriptional profile (Ammon-Treiber and Hollt, 2005; Rhodes and Crabbe, 2005; McClung and Nestler, 2008).
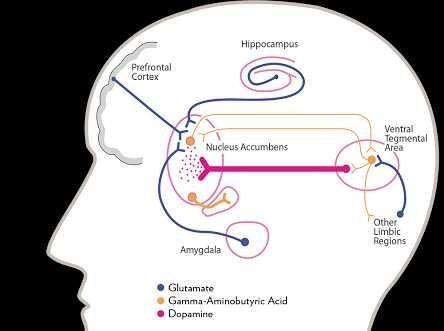
2.1.1 Brain Circuits of )
Brain circuits are beginning to be understood in animals, though there are little supporting data from human beings. The primary circuit seems to be the dopamine pathway that runs from the ventral tegmental area (VTA) through the nucleus accumbens to the prefrontal cortex (Koob, 2001). Dopamine release in either the nucleus accumbens, prefrontal cortex, or both is produced by all misused drugs apart from the benzodiazepines (Chiara et al., 1997). Some (for example, cocaine) act on the dopamine terminals, whereas others (eg, opioids) increase cell firing at the level of the cell bodies. Direct injection of drugs into these brain regions is reinforcing since animals will administer opioids and cocaine directly into them. Moreover, drug withdrawal is associated with reduced dopamine transmission in these regions and aversive drugs (eg, K agonists) inhibit dopamine release there. Paradoxically some other aversive experiences such as pain cause dopamine release, and some argue that changes in this transmitter reflect not simply there in forcing actions of drugs but their salience as a conditioned cue. The feasibility of measuring dopamine release in human brain by displacement of radioligands has already been mentioned and requires exploration. Other brain regions important in addiction are the globus pallidus and the amygdala (both of which receive projections from the nucleus accumbens), and the monoaminergic nuclei of locus coeruleus and raphe. Significant changes in transmitter function in these regions have been found with opioids and stimulants in thalamus (Pratt, 1991).
Fig 1; Brain system and addiction (Robinson and Berridge, 2008)
Brain systems and drug addiction Parsimonious theories have attempted to explain how and why addiction occurs. One of the major theories asserts that overall enjoyment of life and pleasure-seeking behaviors push drug abusers to use higher doses of drugs to improve mood and to cope with physical and/or emotional pain. However, the scientific way to explain addiction is to consider its biological component as hypothesized by the incentive-sensitization theory. Research in humans and animals demonstrates that repeated drug use changes the brain of addicts in progressive, persistent and complex ways (Robinson and Berridge, 2008). The incentive-sensitization theory asserts that the motivational properties of drugs are related directly to their subjective pleasurable effects which create adaptations in the brain (Robinson and Berridge, 2000). In an evolutionary biology perspective, early study suggested that pleasure was associated with an event that contributed to ensure the survival of species called beneception (Troland, 2001). Thus, it is well-known that the brain reward system evolved to ensure activities essential to species survival, such as sexual activity and feeding behaviors and that dopamine regulates pleasure and reward in this system. Unfortunately, the evolutionary processes that attached pleasure and reward to advantageous behaviors also reinforced negative ones.
2.1.2 Dopamine system
Addiction to all major classes of abused drugs has been linked to increased dopamine (DA) transmission in the same parts of the brain associated with normal reward processing. Drug of abuse often induce elevated levels of neural dopamine release in an extremely convenient way, causing the reward system to become flooded with it and reinforcing the addictive cycle (Kelley and Berridge, 2002; Bressan and Crippa, 2005). All addictive drugs elicit the excitation of the dopaminergic neurons in the ventral tegmental area (VTA) of the midbrain and in the shell of the nucleus accumbens (NAcc) (Nestler, 2005). New research shows that the mechanisms underlying addiction predisposition occur in a reward pathway of the brain (Casey et al., 2014). In fact, there is emerging evidence that having a reduced dopamine response to drugs is a high-risk factor for developing addiction in humans (Casey et al., 2014). Importantly, repetitive substance abuse produces the activation of the reward pathways in the brain in unusual way causing neurophysiological and neuroplastic changes (Ross and Peselow, 2009). This unnaturally elevated levels release of dopamine in reward system is associate with the generation of the brain derived neurotrophic factor (BDNF) as a compensatory mechanism to deal with oxidative stress in dopaminergic neurons (Vargas-Perez et al., 2014). This suppressive effect of BDNF on dopamine unnatural release causes the pain, distress and withdrawal symptoms (Vargas-Perez et al., 2014). It is interesting to mention that the way BDNF regulates addiction depends greatly on the drug type, the brain region and the phase of addiction (Koo et al., 2012; Lin and Wolfn, 2015). At the receptor level, D2/D3 receptors stimulation in the striatum and other areas is linked to sensitization, drug addiction and relapse (Wise and Koob, 2014; Lee et al., 2009), with a significant decrease in D2 receptor availability in dorsal and ventral striatum induced by the drug that persist months after protracted detoxification (Volkow et al., 2011)
2.1.3 Interoceptive Insula System
Insular cortex has considerable morphologic variability among mammals. These differences could be related to its multiple cognitive, affective-chemosensory and sensorimotor functions, associated with dorsoanterior, ventroanterior, and posterior regions of the insula respectively (Butti and Hof, 2010; Yates, 2012; Chang et al., 2013). Recently, human brain lesions and neuroimaging studies (both structural and functional) as well as animal literature highlighted the key role that insula plays in addiction (Naqvi et al., 2014; Droutman et al., 2015). Substantial evidence proposed the anterior insula, a substrate for the capacity of self-awareness and the processing of interoception (Gschwind and Picard, 2014), to be the neural basis for intense urges and decision-making processes that involve certain risks and rewards in smokers (Brody et al., 2007; Naqvi et al., 2007; Zhang et al., 2011; Engelmann et al., 2012; Sutherland et al., 2013; Carroll et al., 2014; Maria et al., 2014; Morales et al., 2014). In cocaine-dependent subjects, numerous studies have reported a gray matter volume reduction in the insula (Franklin et al., 2002; Mackey and Paulus, 2013). Animal modeling studies are also consistent with human studies (Contreras et al., 2007, 2012; Forget et al., 2010; Li et al., 2013; Pushparaj et al., 2013). It has been suggested that the representation of interoceptive aspects of drug use relevant to goal-directed reward seeking is the insula main function in addiction (Naqvi and Bechara, 2010).
2.1.4 Serotonin System
The serotonin (5-HT) system is one of the oldest neurotransmitter/ hormone systems in evolution, probably as old as 800 million years (Mengod et al., 2010). There is considerable evidence that this system is implicated in the vulnerability and establishment of drug use-associated behaviors as well as the transition and maintenance of addiction (Müller et al., 2010; Kirby et al., 2011; Müller and Homberg, 2015). Analysis of animal and human studies have shown that drugs of addiction profoundly change the extracellular 5-HT activity and 5-HT receptor function as well as the organization of brain circuitry by direct and indirect interaction depending on the type of drugs (Müller et al., 2010). It has been suggested that a genetic predisposition may anticipate these serotonergic adaptations and then increasing vulnerability to drug use-associated behaviors. In fact, it has been reported that polymorphisms in the gene encoding the 5-HT transporter is associated with alcoholism in non-human and human primates (Todkar et al., 2013) as well as with sociopathy in alcoholics (Herman et al., 2011).
2.1.5 Stress System
In line with the view that stress contributes to the maintenance of addiction, researchers highlight the role of stress system in vulnerability to drug addiction and relapse through five major components: corticotrophin-releasing factor (CRF), norepinephrine and dynorphin/KOR, orexin and vasopressin systems (Shaham et al., 2000; Shalev et al., 2002; Koob et al., 2014; Volkow and Morales, 2015). In humans and animal brains, CRF receptors are widely distributed, and the major illicit drugs can enhance the release of CRF in the central nucleus of the amygdale (Edwards and Koob, 2010; Wise and Koob, 2014). It has been proposed that exposure to stressors induces the stimulation of the reward system through the enhancement of CRF activity which is involved in relapse behavior (Bruchas et al., 2010). In addition, the CRF system is suggested to control the hypothalamic-pituitary adrenal response to stressors, which contributes impulsivity associated with adolescent drug taking (Koob, 2007, 2008, 2015; Sinha, 2008). Numerous studies established a significant relationship between drug use and norepinephrine stress-responsive system (Erb et al., 2000; Xu et al., 2000; Dunn et al., 2004; Sulzer et al., 2005). All major drugs of abuse act by blocking norepinephrine reuptake and increasing norepinephrine release into the synaptic cleft, or both, which leads to increasing their availability to act upon post-synaptic receptors (Rothman et al., 2001; Sulzer et al., 2005; Weinshenker and Schroeder, 2007). Interestingly, a direct interaction between Corticotrotrophin Releasing Factor (CRF) and norepinephrine systems in mediating the negative affect associated with stress and increasing the rewarding effects of abused drugs has been suggested. In fact, it has been shown that CRF and norepinephrine systems coexist closely in the amygdala (Dunn et al., 2004; Smith and Aston-Jones, 2008) with direct synaptic interactions (Kravets et al., 2015), and regulate each other's expression levels and synaptic release thought direct synaptic interactions (Koob, 1999; Reyes et al., 2011; Kravets et al., 2015). In addition, the dynorphin/kappa opioid system is well known to contribute to the effects of stress on drug consumption. Results from different studies indicated that the release of dynorphin induced by stress exposure, activate preferentially kappa opioid receptors (kOR) and subsequently increased the rewarding values of drugs (McLaughlin et al., 2003; Valdez et al., 2007; Redila and Chavkin, 2008; Ehrich et al., 2014). Anatomical evidence indicates that CRF and dynorphin co-localize to a large extent in the central nucleus of the amygdala (Van Bockstaele et al., 1998; Marchant et al., 2007; Reyes et al., 2008; Van Bockstaele et al., 2010) and reciprocally activate one another (Land et al., 2008; McLaughlin et al., 2003; Nikolarakis et al., 1986; Buckingham Cooper, 1986). Importantly, a recent study reported that in response to stress, norepinephrine modulation may influence significantly the dynorphin and CRF circuitry, and in turn, the activation of CRF and dynorphin neuron in the amygdala by noradrenergic afferents may regulate the release of norepinephrine via projections to the locus coeruleus (Kravets et al., 2015). In addition, accumulating evidence highlights the importance of orexin system in drug-seeking behavior, especially cue-induced relapse and stress through the activation of stress pathways in the brain but not by drug itself (Ebrahimian et al., 2016; Sakurai, 2014). A reciprocal activation was previously suggested between CRF and orexin systems (Winsky-Sommerer et al., 2004; Sakamoto et al., 2004). This interaction is pharmacologically significant as reported recently by Navarro et al., 2015 and may explain stress-induced relapse in former cocaine users. In addition, orexin receptors have been identified as potentially important targets for anti-relapse treatment (Zhou et al., 2011). There is in fact considerable evidence that orexin receptor antagonists might be effective at blocking addiction-related behaviors (Yeoh et al., 2014). Over the last few decades, the potential role of vasopressin system as a mediator of the drug's effects has been suggested (Koob, 2008; Bisagno and Cadet, 2014; Buisman-Pijlman et al., 2014; Zhou and Leri, 2016). Vasopressin is genetically and structurally related to oxytocin (Goodson et al., 2012), and functionally proposed to play an important role in drug addiction; from drug seeking and drug taking behaviors to withdrawal (Zhou et al., 2008; Rodríguez-Borrero et al., 2010). Zhou et al., 2008 indicated that stress-induced hypothalamic– pituitary–adrenal (HPA) axis activation is coordinated by vasopressin that involves processing and release of HPA related peptides (e.g. ACTH and POMC). Moreover, administration of a vasopressin receptor antagonist in animals was shown to partially alter the drug seeking behavior, as well as prevent reinstatement of drug seeking behavior after abstinence (Subiah et al., 2012; Zhou et al., 2008). Hence, it may be important to explore the value of vasopressin receptor antagonists in implementing treatment and management strategies for drugs abuse and prevention of relapse (Zhou and Leri, 2016). It's important to note that all the stress systems compounds cited here are interconnected with several reward-related macro-systems including meso-corticolimbic dopamine and extended amygdala, which confer them an ideal position for the modulation of addiction-related behaviors and reward processing.
2.2 EFFECT OF NEUROTRANSMITTER
Dopamine
Meso-corticolimbic dopamine system Dopamine neurotransmission in the nucleus accumbens has been suggested to be important in the reinforcing effects of the drugs. Thus, the levels of dopamine have been measured in AA and ANA rats after intraperitoneal ethanol administration (Kiianmaa et al., 1995) and after voluntary ethanol intake (Nurmi et al., 1996) Whether a theory puts dopamine neurotransmission into mediating rewarding effects of drugs or determining their incentive salience (a motivation to seek and take), a projection from dopamine cells in the ventral tegmental area to nucleus accumbens is invariably implicated. This pathway is a main part of mesolimbic dopamine system. Drugs of abuse share an ability to intervene with this system by altering the activity of the dopaminergic cells directly (Kahlig and Galli, 2003; Elliott and Beveridge, 2005), or indirectly through other neurotransmitter systems. The dopamine cells also send projections to the prefrontal cortex, amygdala, olfactory tubercle and caudate-putamen (Bardo, 1998). These areas together with some other nuclei and their interconnections form a so-called motive circuit that is involved in the translation of biologically relevant environmental and pharmacological stimuli into adaptive motor responses (Pierce and Kalivas, 1997).
Fig 2; Schematic drawing of the connections between ventral tegmentalarea (VTA), nucleus accumbens (NAc) and prefrontal cortex (PFC). DA = dopamine, D1 = dopamine D1 receptor, Glu = glutamate (Klitenick et al., 1992; Carr and Sesack, 2000).
Drugs of abuse elevate the extracellular levels of dopamine in the nucleus accumbens (Di Chiara and Imperato, 1988; Leone et al., 1991; Kiianmaa et al., 1994). The mechanism behind this increase depends on the drug. Psychostimulants prevent the uptake of dopamine from the synaptic cleft and induce release from synaptic vesicles without electrical stimulation of the neuron (Pierce and Kumaresan, 2006). Ethanol increases the firing rate of dopamine neurons in the VTA either because of a direct action on these cells or through GABAergic, glutamatergic or opioidergic mechanisms. Opioids are known to bind to µ-opioid receptors in GABAergic interneurons in the ventral tegmental area and by inhibiting the activity of these neurons they disinhibit the dopamine cells (Johnson and North, 1992). Opioid induced elevation of extracellular dopamine levels in the nucleus accumbens, however, can also be a result of opioids binding to receptors within the nucleus accumbens. In addition, dopamine-independent mechanisms have been suggested to participate in opioid reinforcement. Interactions within so-called extended amygdala (Pierce and Kalivas, 1997), specifically in the prefrontal cortex, hippocampus, amygdala and ventral pallidum may be important (Bardo, 1998; Caille and Parsons, 2004; Sotomayor et al., 2005). Besides that, acute administration of drugs enhances dopamine transmission, repeated exposure to them tends to augment this effect and makes the dopaminergic pathways hyper responsive to many drug effects (Vanderschuren and Kalivas, 2000). Dopamine receptors. Two families of G-protein coupled dopamine receptors are found in the brain (Lachowicz and Sibley, 1997). The receptor types D1 and D5 belong to the D1 -family and D2, D3, and D4 to the D2 -family. The activation of D1 -family of receptors stimulates the enzyme adenylate cyclase, while the receptors In the D2 -family are inhibitory. Dopamine receptors are found both pre- and post-synaptically. Thus, depending on the receptor type and location increased dopaminergic tone can lead to either augmented or attenuated activity of the target neurons.
2.2.1 Interactions of ethanol and opioid systems
Signs of concurrent alcohol and tramadol use can seem mixed, because both drugs affect the central nervous system. As a painkiller, patients taking tramadol may seem subdued and euphoric with a reduction of anxiety. At high dosages, people using tramadol will have distorted perceptions of pain (Traynor et al., 2008). According to Patterson (2015), symptoms that can present when mixing tramadol and alcohol include:
· Abdominal problems.
· Vertigo.
· Loss of coordination.
· Memory loss.
· Lethargy.
· Irregular breathing.
· Seizures
Individuals who constantly abuse Tramadol will experience prolonged periods of feeling less pain, but as the body becomes more accustomed to tramadol, it will take higher amounts to get the initial effects. This is called developing a tolerance to the substance, and the same can occur with alcohol (Bush, 2015). Beyond the physical and behavioral symptoms mentioned above, use of tramadol and alcohol are highly associated with addiction and dependence. This becomes even more problematic when combined with tolerance to the substances individually or together (Sacks et al., 2015). As larger amounts of the substances are needed, the brain becomes more familiar with the effects and begins to require them to function normally. This phenomenon is called dependence. The development of tolerance and dependence fuels addiction. Addiction refers to the compulsive desire to seek out and use the substances even when negative outcomes are probable (Center for Disease Control and Prevention, Fact Sheets- Alcohol Use and Your Health, 2015). Concurrent alcohol and tramadol problems are quite serious and can result in death. Individuals abusing elevated levels of alcohol or tramadol together should be immediately taken to a hospital. Together, the drugs can cause: Taking both alcohol and tramadol increases the potential for a drug overdose, as the combination modifies the individual effects of the substances (Inciardi et al., 2006). Unfortunately, alcohol is commonly abused with tramadol, which enhances the sedative effects of each, leading to an increased risk for life-threatening effects
2.3 Tramadol
Fig 3. Schematic structure of Tramadol (Adams et al., 2006)
Tramadol is a centrally acting analgesic for the prevention and treatment of moderate to severe pain in acute or chronic conditions. As with all other analgesic drugs, the dose of tramadol should be adjusted according to the severity of the pain and the individual sensitivity of the patient. Unless otherwise prescribed by the physician, the patient receives 50–100 mg tramadol (1.5 mg:kg: day based on a 60-kg person) three to four times daily. A daily dose of more than 400 mg is usually not necessary. Tramadol is (1RS;2RS) -2- (dimethylaminomethyl)-1-(3-methoxyphenyl)-cyclohexanol hydrochloride. The compound is manufactured as a 1:1 ratio of two enantiomers which contribute to the antinociceptive effects of tramadol by different modes of action. The effects of the two enantiomers complement each other as the ()-enantiomer is the main opioid component but also enhances serotonin release, whereas the ()-enantiomer inhibits noradrenalin uptake. The combination of these actions in the racemic compound tramadol produces effective analgesia (Raffa et al., 1992, 1993). Further details concerning the influence of tramadol on neurotransmitter systems of the brain are reported by (Frink et al., 1996). In an update of earlier toxicological data presented by (Lagler et al., 1978), in the following overview newer preclinical toxicological investigations with tramadol are evaluated.
2.3.1 Clinical Use of Tramadol
The blend of efficacy, multiple formulations and a low potential for serious adverse effects at higher doses or in prolonged therapy of tramadol hydrochloride provide clinicians with a useful analgesic for short- and long-term use in the hospital and community settings. The two enantiomers of tramadol, and its principle metabolite O-desmethyltramadol, produce relief of moderate to severe pain across the range of acute and chronic pain states by combining synergistically weak opioid and monoaminergically-mediated antinociceptive mechanisms (Bamigbade and Langford, 2001). Up to a dose of 400 mg/day tramadol has an efficacy equivalent to codeine, dextropropoxyphene, paracetamol and aspirin oral combination analgesics (Bamigbade and Langford, 2001). Tramadol is demonstrably inferior to morphine in severe pain; however, it is free of some of the clinically significant adverse effects seen with other opioid analgesics of similar efficacy, particularly respiratory depression. Tramadol causes minimal dependence and tolerance and has very low abuse potential; consequently, it is not scheduled as a controlled drug. Tramadol also lacks the prostaglandin inhibition-mediated adverse effects of the non-steroidal anti-inflammatory drugs. Although data reflect 20 years of clinical experience with tramadol, intraoperative experience remains limited due to historical concerns of accidental awareness with an outdated anesthetic technique that has superseded (Bamigbade and Langford, 2001).
Fantastic article! This is such a heavy subject right now and the problem is only getting worse and far more dangerous with Fentanyl and Carfentanyl.
I have a very close friend who is being prescribed more and more to him every month, even though they aren't necessary. It just goes to show the people we trust to keep us in good health aren't working for us at all. In fact the truth if it all Is that its all about reaching a quota given to the Doctors by ''big pharma''.
Its absolutely sickening.
I've followed and up-voted your post I look forward to your future content!
Im unsure if it is of any interest to you, but For info and technical and fundamental analysis of crypto's, an insight into how the whales manipulate the market and how to get involved in an exciting 12 month millionaire challenge we've recently started, check out @cryptobroye :)
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://www.sciencedirect.com/science/article/pii/S0091305716301046
Thank you henriano for making a transfer to me for an upvote of 1.85% on this post!
Half of your bid goes to @budgets which funds growth projects for Steem like our top 25 posts on Steem!
The other half helps holders of Steem power earn about 60% APR on a delegation to me!
For help, will you please visit https://jerrybanfield.com/contact/ because I check my discord server daily?
To learn more about Steem, will you please use http://steem.guide/ because this URL forwards to my most recently updated complete Steem tutorial?
abuse