Second law of thermodynamics: Heat Engine
The thermal machines base their operation on the second principle of thermodynamics. These machines burn a fuel that produces an increase of internal energy and mechanical work. The higher the temperature, the greater the amount of energy that can be transformed into work. In order to be able to use them they must have a cyclic operation. This is achieved by continuous combustion. Not all energy becomes useful work. The thermal efficiency indicates the amount of energy that is transformed into work. It is expressed in percentage and is always less than 100%. Next we will see a more detailed development of this interesting concept and its operation.
The thermal machines constitute one of the most surprising achievements of modern technology, since through them it has been possible to obtain abundant and cheap mechanical energy, to move all kinds of industrial and transport machinery, which allow us to comfortably satisfy the complex needs of the current life, practically without physical effort on our part. The formidable development that has had the industry, agriculture, communications and other sources of satisfactors, would not have been possible without the essential driving force provided by the thermal machines, which day by day multiply in size and number to supply a demand always growing. It is necessary to clarify, nevertheless, that the thermal machines are not the only sources of motive power that is counted at present.
Source
There are other sources such as hydraulic machines, wind and other minor. However, thermal machines contribute around 75% of all the mechanical energy consumed in the world and have the enormous advantage of being autonomous, which makes them irreplaceable in the field of transport.
Another amazing fact about thermal machines is that they produce motive power from heat as raw material. Heat is a source of primary energy, known since the dawn of civilization, always related to temperature and the remarkable effects it produces on the bodies to which it is applied, particularly food. But heat is a subtle, immaterial form of energy that is not usually related to the tangible and material concept of movement and the work produced by the motive force. Hence the extraordinary fact that thermal machines can transform that subtle and impalpable energy into something as real and perceptible as mechanical energy.
Second law of thermodynamics
It is one of the most important laws of physics; even though it can be formulated in many ways, it all leads to the explanation of the concept of irreversibility and entropy. This last concept, when treated by other branches of physics, especially by statistical mechanics and information theory, is linked to the degree of disorder of matter and the energy of a system. Thermodynamics, on the other hand, does not offer a physical explanation of entropy, which is associated with the amount of non-usable energy of a system.

Source
The second principle of thermodynamics states that although matter and energy cannot be created or destroyed, but are transformed, and establishes the sense in which this transformation occurs. However, the capital point of the second principle is that, as with all thermodynamic theory, it refers only and exclusively to states of equilibrium. Any definition, corollary or concept extracted from it can only be applied to states of equilibrium, so, formally, parameters such as temperature or entropy itself will be defined only for states of equilibrium. Thus, according to the second principle, when you have a system that goes from a state of equilibrium A to another B, the amount of entropy in the state of equilibrium B will be the maximum possible, and inevitably greater than that of the state of equilibrium A. Obviously, the system will only do work when it is in transit from steady state A to B and not when it is in one of these states.
The formal definition of the second principle of thermodynamics states that:
In a state of equilibrium, the values that take the characteristic parameters of a closed thermodynamic system are such that they maximize the value of a certain magnitude that is a function of said parameters, called entropy.
The second principle of thermodynamics states that said entropy can only be defined for states of thermodynamic equilibrium, and that among all the possible equilibrium states -which will be defined by the characteristic parameters-, only the one, of all of them, can be given, it maximizes entropy.
The consequences of this statement are subtle: when considering a closed system aimed at equilibrium, the possible equilibrium states include all those that are compatible with the limits or contours of the system. Among them is evidently the equilibrium state of departure. If the system varies its state of equilibrium from the starting point to another, this is because the entropy of the new state is greater than that of the initial state; if the system changes its equilibrium state, its entropy can only increase. Therefore, the entropy of a thermodynamically isolated system can only increase. Assuming that the universe started from a state of equilibrium, that in every moment of time the universe does not move too far away from thermodynamic equilibrium and that the universe is an isolated system.
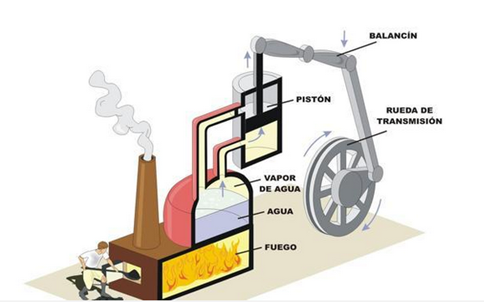
Visually, the second principle can be expressed by imagining a boiler of a steamboat. This could not produce work if it were not because the steam is at high temperatures and pressure compared to the environment that surrounds it.
Heat Engine
The heat engine belong to the group of compressible fluid. That is, those that have the ability to perform an exchange of mechanical energy through a fluid that manages to cross them. On the other hand, the process itself may have several variants. That is, if the procedure gets the fluid to increase its own energy, then the machinery will receive the name of generator, whose most relevant examples are compressors and pumps. On the other hand, if the fluid significantly decreases its energy, then it is called the motor, where the turbines and the explosion engines are located.
Source
Due to these variants in the process, they can also be classified in relation to the form of compressibility of the fluid in question. Then we can find several types of thermal machines. One of these types is the hydraulic, which operates only with fluids considered incomprehensible. Within this group it is possible to highlight the machines that operate with liquids such as water, while some models also work with gases, just when they behave with that extra degree of incomprehensibility, being an important example the fan.
Also, the energy that they take advantage of is only the mechanics, which is available in the same fluid, as in the case of kinetics and potential. That is why if at any given moment the temperature level of the fluid at the entrance of the machinery itself is increased, then at the exit of this one a much hotter fluid can be obtained, without that change of temperature necessarily means a benefit Greater energy. This is how the mills, for example, make use of the energy of the water currents, while the hydroelectric plants take advantage of that potential that is in the water, when it is embalmed.
Another example that we can mention within the group of machines of understandable fluid are those machines qualified as volumetric or as positive displacement. It is a class that can be traversed by a known fluid. These, in turn, can be subdivided into two groups: the rotating or the alternatives, this will always depend on the function of the movement that can be obtained. On the other hand, those that are crossed by a continuous fluid receive another name, they are turbo machines, which also cannot be sub classified since they are always rotating.
Models of thermal machines
Now, once determined the own models of the understandable fluid machineries, we go to define the own characteristics of one of the exponents of this group: the thermal machines. This class operates with such fluids, regardless of whether they are condensable like thermal steam or non-condensable machines, such as gas turbines. Here what happens is that there is an advantage of the thermal energy of the fluid, fundamentally because the mechanical energy is obtained thanks to the expansion of the fluid, that is, thanks to the fact that it manages to increase its specific volume. What happens is that when the temperature level of the fluid increases at the moment of entering the machine, then later a greater amount of mechanical energy will be able to be obtained on the same axis of the machine. It can be said that thermodynamics has been concerned with the study of the exchanges of energy produced in thermal machines. Just as many groups have different models; this case is not the exception.
We can classify thermal machines with two fundamental criteria in mind: the amount of fluid handled and the movement that the machine will carry out. In the case of thermal engines, for example, the energy of the fluid that will pass through the machinery will decrease considerably, which is why mechanical energy is obtained. However, the same does not happen with thermal generators, which present an inverse process. In this way, the fluid will increase the energy at the moment in which it passes through the machine.

Source
The thermal motors, in addition, are in themselves machineries, since they use the energy that has been the result of a combustion process; always with the objective that energy is generated from the fluid that is going to be used in later instances to obtain, precisely, mechanical energy. For all this, certain cycles called thermodynamic, which need the use of a generating group that can be hydraulic or thermal. In the first case, it is related to the steam turbine cycles. In the second case, it is linked to the gas turbine cycles. Due to this, if the generator is absent, then the motor group will not be able to function properly.
History of Heat Engine
Today it is common to think that in the complex process of creation, assimilation and application of scientific knowledge, technology is the last stage that emanates from scientific research. While it is true that there is a complicated interrelation between science and technology, to the extent that it is difficult to think that the latter is alien to scientific work, it was not always so. It is true that, for example, communications, wired and wireless arise from the understanding of the behavior of the electromagnetic field through the studies of Faraday, Maxwell and Hertz in the second half of the last century. Thus, a technology emanated from the results of scientific research. But in the case of devices that transform energy and in particular thermal energy into mechanical work, the situation was completely the opposite. These last devices, which we will now call thermal machines, were developed from their most incipient form, in the eighteenth century, to practically the way we know them today, which happened already towards the middle of the 19th century, without the existence of the least understanding about the theoretical causes, that is, the scientific explanation of its functioning. So let's do a little history.
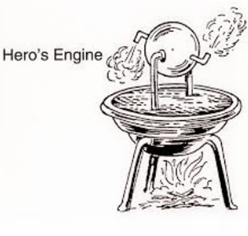
The first thermal machine of which we have written evidence was discovered by Hero of Alexandria (~ 130 a.C.) and called the aeolipila. It is a primitive steam turbine consisting of a hollow globe supported by a pivot so that it can rotate around a pair of stumps, one of them hollow. Water vapor can be injected by said stump, which escapes from the balloon to the outside by two tubes bent and tangentially oriented in opposite directions and placed at the ends of the diameter perpendicular to the axis of the balloon. When steam is expelled, the balloon reacts to this force and rotates around its axis.
Source
In the same work of Hero the first prototype of a pressure machine is also described, which later was the subject of several studies by Matthesuis in Germany in l57l, of Caus in France in 1615 and in Italy by Ramelli in 1588, della Porta in 1601 and Branca in 1629. Later, in 1663, Edward Somerset, the second Marquis of Worcester, in his book A Century of Inventions describes a method to raise a volume of water using steam. His description is obscure and lacks drawings; and the question remains whether he built the machine or not. It was not until the years of 1698 to 1725 that the idea of Somerset was put into practice and used to meet diverse needs. In 1698 Thomas Savery obtained a patent for a machine used to raise considerable amounts of water. Its operation consisted essentially of injecting steam into a container filled with water until its contents were emptied through a vertical tube through a safety valve. When the container is empty, the steam supply ceases and the contained vapor condenses by means of a jet of cold water that falls on the outer walls of said container and that comes from a cistern placed in its upper part. This produces a vacuum and allows another tube, controlled by another safety valve, to draw water from the distributor well to whatever the source. Meanwhile, a parallel operation is carried out in another container similar to the first one.
The steam is supplied from an oven consisting of a main boiler that has a continuous supply of hot water which comes from another oven that heats cold water by the fire lit in its fire. The water levels in the boilers are controlled by two pressure valves. This machine, which can be considered as the first steam engine, found considerable use in the extraction of water from coal mines and in the distribution of water for houses and small communities. This machine was subsequently modified in various ways, all designed to improve the amount of water and the height at which it could rise, since these characteristics were limited by the pressure that the boilers could withstand. As early as 1690 Denis Papin had suggested that vapor condensation should be used to produce a vacuum under a piston that had previously been raised by the action of steam.
This was the first version of a steam engine using a cylinder and a piston. In 1705 Thomas Newcomen and John Cawley, his assistant, improved the operation of the piston by forcing its fall by action of atmospheric pressure. In doing so, it produced mechanical work on a pump that introduced the water to be pumped. After several technical adjustments these machines were produced in large size and in series by John Smeaton until in 1770 they were surpassed by the innovations due to James Watt.
In 1763 this remarkable Scottish instrument manufacturer, when repairing one of the Newcomen machines, was surprised to see the enormous waste of steam that occurred during the heating and cooling process of the cylinder, inside which the piston operated. The remedy, in his own words, would be to keep the cylinder as hot as the incoming vapor. After six years his experiments led him to patent, in 1769, a machine that exceeded those of his predecessor for its greater speed in the piston stroke and for being much more economical in terms of fuel consumption; however it was reduced to pumping and suffered from other technical limitations. The way in which these limitations were overcome is out of context, but it is worth noting that Watt himself in 1781 devised the way to use the machine to rotate an axis and therefore open its applications to many other uses besides the pumping. In the hands of notable inventors such as Trevitchik and Woolf in England, Evans in the USA, Cugnot in France and others, this machine reached a state of perfection such that in 1829 George Stephenson was the first to adapt it to a locomotive essentially in the same way used by today's heaviest locomotives.
Also, in 1802 it was used for the first time by W. Symington to navigate the Charlotte Dundas trailer. Later, in 1807, the North American Robert Fulton navigated a boat on the Hudson River with steam engines designed by Boulton and Watt. Between those years and the last few years of the last century, with improvements in design and construction, the steam engine became the usual machine for marine navigation, achieving very high vapor pressures and considerable piston speeds. With the invention of the steam turbine, marine navigation acquired its maximum degree of development, only later overcome by the advent of nuclear fuels. In the steam turbine, developed by Parsons in 1884 and perfected by Laval in 1889, the steam pressure is used to directly put the fluid in motion and not the piston.
In all this process of inventions and innovations the inventors hardly had a theory, like the electromagnetic one in the case of the radio that will guide them in their way.
The thermometers product of the work of Gabriel Fahrenheit in 1717, were reproducible with a high degree of precision and arose from the need to subsist with a more precise instrument the sensations of cold and hot that to the touch are difficult to quantify. In fact, long before its construction, scientists like Leonardo da Vinci, Galileo and others knew that on contact with a third body, usually the air, two or more bodies in contact with it "mixed in an appropriate manner" until reaching a same "condition". Hence the word temperature that comes from the Latin temperate which means "mix appropriately" or temper. But for no one was it clear what mechanism was implicit in that mixing.
For more information, I invite you to visit the following links: