GENE THERAPY IN HUMANS AND RELATED CONCERNS
In August 2017 United States Food and Drug Administration (USFDA) approved Yescarta (Axicabtagene ciloleucel) therapy to treat adults with certain types of large B-Cell lymphoma (a type of blood cancer). Yescarta uses chimeric antigen receptor (CAR) T-cell therapy for treatment.Last month of previous year FDA approved Luxturna, first in vivo gene therapy, for the management of blindness due to Leber'scongenital amaurosis.
Gendicine, first commercial gene therapy, was approved in China for the treatment of certain cancers. In 2011 Neovasculgen, registered in Russia as the first-in-class gene-therapy drug for treatment of systemic peripheral artery disease, including limb is chemical. In 2012 Glybera, became the first treatment to be approved for clinical use in either the United States and European Union after its endorsement by the European Commission.
What is Gene therapy
A technical manipulation which involves the replacement of defective genes with the normal ones in order to treat genetic disorders. It is an artificial method of introducing the DNA into the cells of human body. First time gene therapy was successfully accomplished in the year 1989, by the National Institutes of Health.
Successful therapeutic use of gene transfer and first direct insertion of human DNA into the nucleus was performed by French Anderson in a trial starting in September 1990.
Gene therapy is precision of the procedure and the intention of direct therapeutic effects.Medical procedures that introduce alterations to a patient's genetic makeup can't be always considered gene therapy.For example bone marrow transplant may lead to the change in genetic make up of the cell but is not intentional, we can't consider it gene therapy.

Gene therapy is classified into two types on the basis of type of cell, the cell on which the therapy is done.
Somatic gene therapy.
Germ line gene therapy.
In formar case the therapeutic genes are transferred into any cell other than a gamete or undifferentiated stem cell and in later case Germ line gene therapy (GGT), sperm or egg cells are modified by the introduction of functional genes into their genome. Thus modified germ cell causes all the organism's cells to contain modified genes.
Why we need gene therapy?
Gene therapy is considered future management of many diseases with worst case prognosis like cancer, viral infections and congenital diseases. Apart from these, potential scope of gene therapy is considered in infertility, gene doping and human genitic engineering. Experiment done on mice has shown that fertility can be restored by using the gene therapy method CRISPR. Gene doping though still hypothetical is a non-therapeutic use of gene therapy by athletes in order to improve their performance in sporting events. It has moral and ethical considerations. Germ line engineering of Human genitic engineering can increase the memory of a person, change its physical appearance, increase life span of an individual and augment the physical strength.Though the committee of the American National Academy of Sciences and National Academy of Medicine gave qualified support to human genome editing in 2017 but only in stringent oversight in serious conditions.
Some Major challenges and unsolved problems!
Shelflife of Therapeutic genes
Before gene therapy can start acting a permanent cure for a condition, the DNA introduced into target cells must remain physiologically active and the cells containing DNA must be stable. Problems start encountering when integrating therapeutic DNA, genome and the rapidly dividing nature of many cells prevent it from achieving long-term treatment goals. So the patient may require multiple treatments plans.
Host body Immune response to foreign genes
Any time a foreign object or material is introduced into human body the immune system is activated to attack the invader. So it becomes obvious that immune system reduces gene therapy effectiveness, if not reject it. The immune system's increased response to viruses that it has seen before reduces the effectiveness and will lead to multiple treatments.
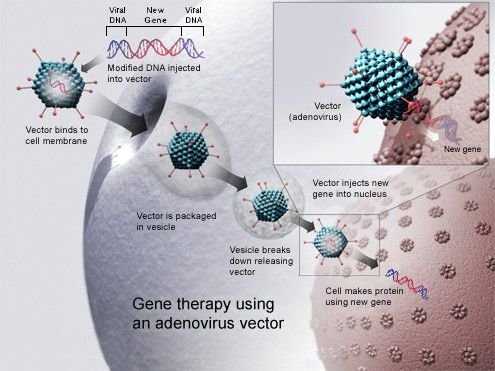
Problems with viral vectors
Vector is a medium that cause delivery of DNA into cells. Mostly used vectors are viruses. Viral vectors have the risks of toxicity, inflammatory responses, gene control and other issues like targeting.
Multigenic disorders
Many disorders are controlled by multiple genes, such as high blood pressure,heart disease, Alzheimer's disease and diabetes militus can be treated with single gene therapy which in turn complicates gene therapy and may need multiple therapies which is impossible and costly. In case of multiple therapies there are chances of breach in the Weismann barrier (barrier which separates somatic and germ-line) protecting the testes, patentially changing the germline, will lead ethical concerns of regulations in many countries that prohibit the said practice.
Insertional mutations to host
Place of integration of therapeutic DNA in the host cell in a sensitive spot in the genome can lead to the process of mutagenesis. For example if the integration happen in place of tumor suppressor gene, the therapy could induce a malignant tumor that can be more dangerous than the disease under treatment. The same has resulted in clinical trials for X-linked severe combined immunodeficiency (X-SCID) disease patients, where hematopoietic stem cells were transduced with a corrective transgene through a retrovirus, and this led to the development of T cell leukemia in 15 percent of patients under clinical trials.One possible solution to this is adding another functional tumor suppressor gene to the DNA to be integrated in host. This leads to another problem, longer the DNA, the harder it is to integrate into cell genomes. CRISPR technology allows researchers to make much more precise and short genome changes at exact locations.
Cost vs benifit ratio
In 2012-2013, Alipogene tiparvovec or Glybera, for example were available at a cost of $1.6 million per patient, was reported in to be the world's most expensive procedure or drug at that time.
Clinical trials and related eaths
The last and the most concerned challenge is deaths happened in clinical trials.At least, hree patients deaths have been reported in gene therapy clinical trials, getting the field trials under close scrutiny. The first one happen to be Jesse Gelsinger in 1999. Jesse Gelsinger died because of immune rejection response. Another X-SCID patient died of leukemia in 2003. In 2007, a rheumatoid arthritis patient died from an infection; though the investigation concluded that the death was not related to gene therapy but because of other systemic disorder.
SOURCES OF CONTENT ;
Hey @yoo1900, this post of mine was selected by you to vote it but because of some reason none of the today's posts got vote still.
I think there is some technical error please check it if you can it will be your greatness.
Also thanks to you from my side choosing my post to upvote. I am one of your first folland continue to be one in future. I appreciate your good work, you do for minnows.
Kindly check the post, none of the selected posts of the day got voted.
You got a 5.26% upvote from @emperorofnaps courtesy of @muzi0202!
Want to promote your posts too? Send 0.05+ SBD or STEEM to @emperorofnaps to receive a share of a full upvote every 2.4 hours...Then go relax and take a nap!
I thing I must tell you is that, you have not set a values of minimum ROI. This makes sometimes suspicious to bid for your bot or not. You have mentioned the maximum ROI about 10 percent. Please upgrade your bot settings if you can.
I suggestions may not be profitable but it raises trust of your users.
Hope you will take a look of my suggestion and consider the settings update.
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://en.wikipedia.org/wiki/Gene_therapy
It took me half a day to write this post and you stupid bot found a similar content on wikipedia article. Yes I had to copy some definitions but we can't change the definition words. If you check the content nothing has been copied directly from Wikipedia or any other website. May be the Wikipedia lines which were also edited by me has some likeness in writing. I hope you consider your comment and check again the website and my post.
Hey there @muzi0202!
It is evident that you have put effort in your article, but there are some paragraphs that resemble a lot with the ones in wikipedia. This is what triggered the cheetah to comment on your post.
I would like to make some observations regarding this article of yours.
Please try to use your own words when taking information from an external source (such as wikipedia)
It is usually better not to use all caps for headlines.
Please read here about how to correctly search for non copyrighted images and how to cite them in your post.
Also, please note that your reference links are not correct. You cannot use as a reference a google search here
When information we write comes from an internet source, it is also important to write the retrieval date.
Feel free to read more stem posts by @steemstem and read the steemstem guidelines
Steemstem is also on discord where everybody is welcome to introduce themselves and be a part of the community!
Thank you for reading my comments and please let me know if you have any questions
Katerina
From the link you mentioned here, I took a definition from there so I thought it might be reasonable to mention. If you check my post, I don't think there is any direct copy paste material or headline from Wikipedia what was cited by this bit. There are reasons to believe, there may be many articles with same content.
What I thought got wrong was the definitions I took but we can't change the words written in paranthesis. There is a Labour behind a scientific definition and we always had to credit them.
In the end I am really thankful to you providing me insight about proper way of writing an article with best possible skill. Your written mentioned article was so educative and helpful perhaps I may next time will comment a single mistake.
To listen to the audio version of this article click on the play image.

Brought to you by @tts. If you find it useful please consider upvote this reply.
Your Post Has Been Featured on @Resteemable!
Feature any Steemit post using resteemit.com!
How It Works:
1. Take Any Steemit URL
2. Erase
https://3. Type
reGet Featured Instantly & Featured Posts are voted every 2.4hrs
Join the Curation Team Here | Vote Resteemable for Witness
Congratulations! This post has been upvoted from the communal account, @minnowsupport, by Muzi0202 from the Minnow Support Project. It's a witness project run by aggroed, ausbitbank, teamsteem, theprophet0, someguy123, neoxian, followbtcnews, and netuoso. The goal is to help Steemit grow by supporting Minnows. Please find us at the Peace, Abundance, and Liberty Network (PALnet) Discord Channel. It's a completely public and open space to all members of the Steemit community who voluntarily choose to be there.
If you would like to delegate to the Minnow Support Project you can do so by clicking on the following links: 50SP, 100SP, 250SP, 500SP, 1000SP, 5000SP.
Be sure to leave at least 50SP undelegated on your account.