Scientific News #3 - Snail-inspired Glue Edition
Scientific News #3

Image Source
Topic #7: Snail-inspired Glue
Previously available adhesives were cytotoxic (superglues), they adhere only weakly (clam-inspired adhesives) or are not suitable for wet environments. Therefore, there is a need for stronger and more universally applicable adhesives, which ideally are even biocompatible in order to allow their use in medicine.
Mooney and his team have developed a family of two-layered glues that resembles snail slime. The design was inspired by a defensive mucus secretion of slugs, which was known to strongly adhere to wet surfaces. Their mimicked glues consist of an adhesive surface and a dissipative matrix. The surface adheres to the substrate through electrostatic interactions, covalent bonds and mutual physical penetration. The matrix enhances the energy dissipation by hysteresis. The following illustration shows the general structure:
To form the new artifical adhesive like a plaster, the authors first produce an alginate-polyacrylamide hydrogel as the dissipative matrix. Its surface is then treated with a bridging polymer (either polyallylamine, chitosan, gelatin or polyethyleneimine). Next, the surface is modified with coupling reagents, like EDC or N-Hydroxy-succinimid (NHS), to facilitate a subsequent carbodiimide coupling reaction.
The final glue sticks to wet surfaces more strongly than previous adhesives:
The adhesion takes place within minutes with skin, liver or even heart! It is independent of the presence and amount of blood and is compatible with dynamic movements in vivo. The developed adhesives are therefore suitable for tissue adhesives and tissue repair as well as wound dressings:
(A) shows the use of their glue as tissue adhesive. One of their glues was adhered to the liver and sustained 14 times its initial length before debonding occured. (B) The potential use as an heart sealant was shown. (C) The use as a hemostatic dressing was also successfully tested on a deep wound on a rat liver. - Illustrations taken from Ref.
This work gave very promising results for potential future applications!
If you want to have a closer look, use this open source reference:
J. Li, A. D. Celiz, D. J. Mooney, et.al: Tough adhesives for diverse wet surfaces, Science, 2017, 375, pp. 378-381
Topic #8: Gold in an unusual oxidation state
Gold complexes are important in homogeneous catalysis and biomedical applications. So far, mainly Au(I) and Au(III) compounds have been isolated in mononuclear form. The possibly crucial and hence interesting intermediate, the Au(II) complex, remained unknown.
Until now!
Katja Heinze and co-workers isolated the Au(II) -Tetraphenylporphyrin complex: Au(TPP). It is sensitive to oxygen, but can be handled under inert conditions without major problems. Au(TPP) can even be dissolved or purified by sublimation. Not only did they demonstrate the oxidation state, but they also demonstrated the reactivity of the compound. Thus, they created a reference and starting point for further investigations on the biological and catalytic activity of gold compounds.
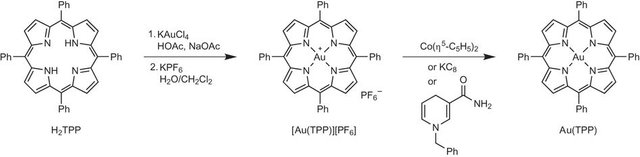(TPP).jpg) Illustration of the synthetic route taken from Ref.
Illustration of the synthetic route taken from Ref.Although gold seems to have the disadvantage of high price at first glance, it is in fact relatively cheap compared to other catalytically active metals such as palladium or platinum and thus interesting for further investigations and applications.
Reference:
S. Preiß, C. Förster, et. al: Structure and reactivity of a mononuclear gold(II) complex, Nature Chemistry, 2017, 9, pp. 1249–1255
Topic #9: Single-molecule experiment on polymer growth
Polymers can be grown through the stepwise reactive addition of monomers to an active site at one end of the polymer, which often is occupied by a catalyst. Whenever you think about such a polymerization process, one intuitively envisions a wormlike polymer growing smoothly and continuously. However, until recently there has been no data on the real time dynamics that underlie the polymerization process. The group around Liu et al. now managed to investigate the growth of a single polymer chain. By attaching one end of a growing polymer to a bead and using magnetic tweezers they managed to visualize real-time polymer growth at a single-polymer level!
The polymer chain is attached to a glass bead (gray surface) and also to a magnetic particle (orange sphere) bound to the Ru-catalyst. The linkage is shown at the top right. By pulling out the chain with a pair of magnetic tweezers, they were able to watch the surprising dynamics of the investigated ring-opening polymerization. - Illustration taken from Ref.
By means of molecular dynamics (MD) computer simulations, the observed stop-and-go growth was attributed to the formation of polymer tangles. It is claimed, that these polymer tangles, called hair balls, form around the catalyst while thousands of monomers are added, until it spontaneously unravels at some point, and then a new hair ball will be formed.
Their work also made it in the "Reseach of the Year" section of the C&EN Journal!
Reference:
C. Liu, K. Kubo, et. al: Single polymer growth dynamics, Science, 2017, 358, 6361, pp. 352-355
or a Review Article of the Science Paper:
S. K. Ritter: Single-molecule experiments reveals polymer growth spurts, C&EN, 2017, 95, 42, p. 4
Economic-scientific Notes
The industrial gases and plant engineering company Linde received an order from Russia worth 1 billion euros! It involves the construction of an olefin plant in the Russian republic of Tatarstan, about 800 km east of Moscow. Starting with the year 2020 the plant is meant to produce plastic precursors. A subsequent strategic cooperation will be established to ensure orders from the Eastern European market. - Ref.
After they met all requirements and received the permission the two chemical giants Dow Chemicals and Dupont merged. The shares of the new company Dowdupont now run under the stock exchange code DWDP. - Ref.
For previously presented topics have a look at:
mountain.phil28