Fantastic Organic Chemistry
Organic chemistry has always been known with life. Chemists at the beginning of the 19th century thought that the compounds produced in organisms were so complex that they could not be artificially produced and they needed a living power to bring these compounds to the field. They said "organic" to this force. In this regard, it is necessary to give them their rights, organic chemistry is life force and nowadays it is used in many areas such as paint, plastic, food, explosive, medicine, petrochemistry.
Well, What Is This Fantastic Organic Chemistry?
Essentially, fantastic organic chemistry has emerged as a subtype of organic chemistry and is a synthesis of the imagination of organic chemistry by organic chemistry laws. The fantastic in its name comes from the purpose of synthesizing compounds which are difficult and sometimes impossible to synthesize. And now, let's have a look at these fantastic interesting products that are increasing day by day from past to present...
Bifluorenylidene Derivatives
It is a new group of molecules synthesized by a group of Spanish scientists wondering how much the double bond can twist without breaking down. The greatest feature of these molecules is that they have the largest double bond synthesized up to now. They have proved that they reach the final point where the double bonds can be twisted by X-ray spectroscopy and ESR (electron spin resonance) spectroscopy, showing that the double bond is singlet spindle and is still a molecule with a double bond in classical sense. While the angle of bending in this aromatic ring, which is never previously connected is 40 degrees, it have reached 66 degrees on the right side and 55 degrees on the left side by chlorine atoms, which allow the molecule to bend more. This is indeed an example of how far to push the limits for chemists.
Catenanes
They are molecules which consist of two or more connected rings like links in a chain. However, the chain here is not a chemical chain in the sense we know. The rings are on side by side like beads but it is not a complete chain. Since there are no solid mechanical connections between the rings of the chain, the rings pass freely to each other. Some chemists have thought of mechanically linking this structure and came up with an interesting molecule. The catenane molecule is formed by the interlocking of two rings containing 26 and 28 carbons.
The red and blue structures represent two different rings with different carbon numbers. Knots can be transformed into catenanes by a mechanism called "strand switch".
For the first time in 1964, the German Schill and Lüttringhaus obtained a catenane as a crystalline powder with a desired chain structure and melting at 125 °C. It consists of two rings of 26 and 28 carbon atoms intertwined. Although this molecule was synthesized for fantasy purposes back then, today it also has two brothers; Rotaxanes and Knots.
The adventure that started with the finding of the use of catenanes in nanotechnology has progressed since 1960 and the synthesis of the first molecular motor was achieved in 1991. Nowadays, more complex molecules with three rings are being studied.
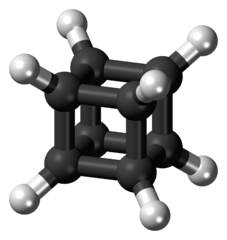
Cubane
It is a cube as its name implies, and the reason for its fantasticness is its determination over what is expected.
The cubane, which is very sensitive to light, water, and air, is degraded only at over 220 degrees and this is not the only interesting feature. While the bond between the two carbons is expected to be 109.5 degrees according to the structure of the compound, the 90 degrees of this angle gives it an incredible solidity. This situation can be explained by the tension energy. Its energy is about 166 kcal/mol. However, it was seen that the expected hybridization of carbon atoms, which is sp3 in shape, does not have sp3 as 31% "s" characterization results.
The difference between the melting point of 130 °C and the boiling point of 133 °C is unusually low. Its another interesting side is that the solid crystal state is not cubic but is rhombohedral. Because it is expected that the crystal structure of a molecule in the form of a cube is also a cuboid. Of course, besides being interesting, it was a beneficial molecule and the chemists who synthesize the molecule were happy with it. It has found usages at some areas such as AIDS, Parkinson and some cancer types by substituting different groups instead of hydrogen. In addition, octanitrocubane, a molecule with twice the explosive power of TNT, is also synthesized from cubane and is likely to be used in many fields of industry.
References
- Kaushik Patel, Ognjen Miljani and J. Fraser Stoddart, Chem. Commun., 2008, 1853
- Journal of Organic Chemistry, 2002, 67 (21), 2002
- Philip E. Eaton and Thomas W. Cole J. Am. Chem. Soc.; 1964; 86(15) pp 3157
- 107 Stories About Chemistry, L. Vlasov, D. Trifonov