Floral Scent - Part 1
I had the great pleasure of working with Prof. Natalia Dudareva while I was employed at Purdue University, trying to elucidate the pathways of synthesis of fragant chemicals produced by angiosperm flowers. These volatile chemicals are released from the flowers primarily to attract insect pollinators, although flower color also plays a critical role in this process. The blend of chemicals released by flowers is often termed "floral scent".
Many flowers, including those of Petunia hybrida, produce volatile floral scent compounds derived from the amino acid phenylalanine. This amino acid is also the precursor of the colored pigments of many flowers, anthocyanins (see my previous post on A colorful experiment with plant pigments). To simplify analyses of the floral scent biosynthesis pathway, we looked specifically at white petunia flowers (Petunia var. Mitchell), which lack the ability to produce anthocyanins.

Natalia Dudareva, Purdue University
In 2004 we developed a method for elucidating these biochemical pathways by feeding excised flower petals with isotopically labeled phenylalanine (specifically with phenylalanine labeled with 5 deuterium atoms on the phenyl ring) indicated with red dots in the image below:

Phenylalanine - Wikipedia, with modifications shown in red
Samples were taken over a time-course, measuring the pool sizes (nmol/g fresh weight) of the internal (endogenous) pools of metabolites, the emission of volatiles into the gas phase (exogenous), and the isotope abundance of these compounds by gas chromatography-mass spectrometry (GC-MS).
As the deuterium-phenylalanine is metabolized, each metabolite derived from deuterium-phenylalanine without modification of the phenyl ring, is labeled with 5 deuterium atoms, and is thus shifted by +5 atomic mass units relative to pre-existing unlabeled molecules, or molecules synthesized from unlabeled-phenylalanine. A molecule containing two phenyl rings (such as benzyl benzoate) can be shifted by either +5 or +10 atomic mass units depending upon the number of labeled rings incorporated. A molecule that has a modified ring structure with two deuterium atoms of the phenyl ring replaced with -OH groups (e.g. eugenol or isoeugenol) may be shifted by only +3 amu.
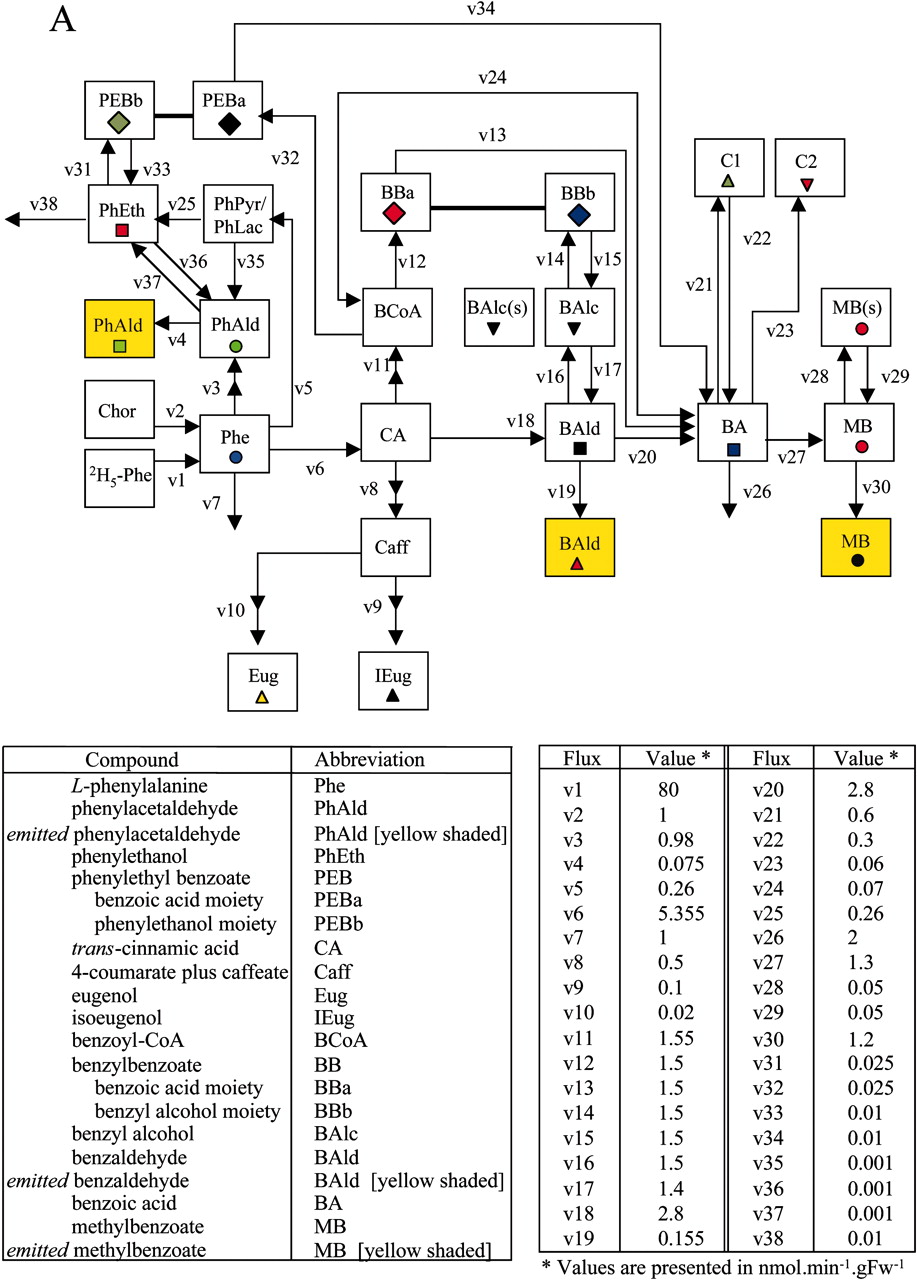
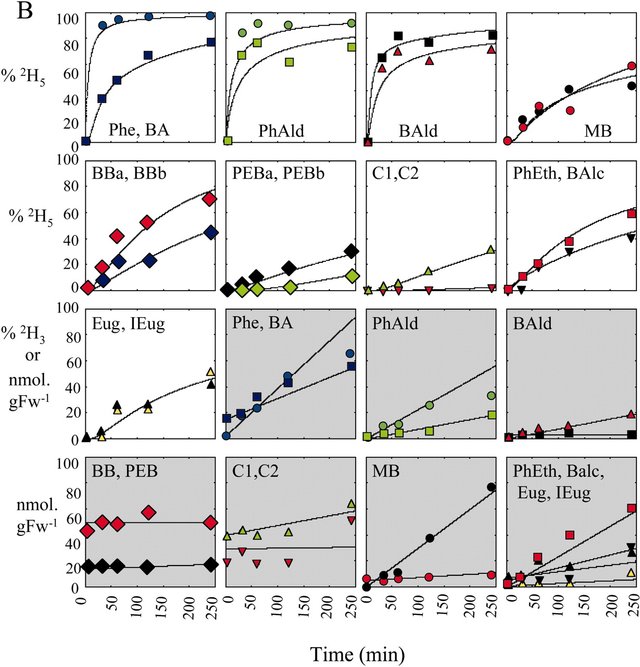
We then developed a computer model (in Visual Basic) that satisfied as many of the observed data points as possible (see Figures A and B below) including isotopic labeling time-courses (graphs with white background in B) and pool sizes (graphs with grey background in B) of endogenous and exogenous metabolites. Figures are drawn from the following article: Boatright, J., Negre,F., Chen, X., Kish, C.M., Wood, B., Peel, G., Orlova, I., Gang, D., Rhodes, D., Dudareva, N. Understanding in vivo benzenoid metabolism in petunia petal tissue. Plant Physiol. 135: 1993–2011 (2004).
In a tedious process, the various metabolic rates (fluxes, designated v1 - v38 in A) were systematically adjusted until a satisfactory fit between the observed (symbols) and computer simulated values (lines) was achieved, as shown in B. The yellow squares in Figure A represent the exogenous pools (i.e. those emitted to the gas phase). The labeling patterns of these emitted (exogenous) metabolites tend to lag behind the labeling patterns of the internal (endogenous) pools of the metabolites, as expected.
These investigations led us to conclude that phenylacetaldehyde must be derived directly from phenylalanine in what appeared to be a single step. This was subsequently confirmed when we identified a single bifunctional enzyme that converts phenylalanine to phenylacetaldehyde:
Our results also suggested that benzyl benzoate seves as a precursor of a major floral scent compound, methyl benzoate, via benzoic acid as an intermediate. This in turn was confirmed using a petunia transgenic mutant that lacked the enzyme that uses benzoyl-CoA and benzyl alcohol to make benzyl benzoate:
Suprisingly this enzyme also catalyzed the synthesis of phenylethyl benzoate. Moreover, the transgenic mutant exhibited a phenotype that suggested increased transport of the plant hormone, auxin. WE TURNED OUT TO BE WRONG ON THE LATTER POINT! Dexter et al. (2008) subsequently showed that this phenotype was most likely due to a ploidy change that occurred during the generation of the transgenic plants that had the PhBPBT gene silenced:
Nevertheless, further progress has been made on refining the metabolic model of floral scent biosynthesis in petunia. This model has now been developed into a kinetic model that incorporates the enzyme properties (Km's and Vmax's) of key enzymes in the network, and that begins to accommodate the dynamic changes in fluxes due to perturbations (such as swelling of the internal pool of phenylalanine, or knock-out of specific genes encoding various enzymes of the network, including phenylalanine-ammonia lyase, the enzyme that converts phenylalanine to cinnamic acid):
Petunia flowers have served as an excellent model system for probing the pathways of synthesis of phenylalanine:
and identification of phenylalanine transport proteins:
Natalia Dudareva, Josh Widhalm, John Morgan and colleagues at Purdue, continue to publish high profile papers on the mechanisms of floral scent emission. One of their latest papers was published in Science, receiving cover page status:
This is a truly fascinating field of plant biochemistry, and I hope to continue to contribute additional articles on this subject matter in the near future. Stay tuned!
If you have any comments, suggestions or questions about this post, please leave a comment below. I will try to respond as soon as possible.