Oscillatory reactions - when chemistry looks alive
Our intuition is based on our daily observations. When we think about chemical reactions, in most cases we are thinking about the "one-way street".
For example, gasoline is ignited in your engine. It releases the energy that perpetuates the car and some gases are coming out from the exhaust.
Or, you scramble the eggs for your breakfast (*according to @abigail-dantes, you need to add some milk as well :) ). During the scrambling, you are changing the proteins and you can't reverse that. Actually, it's "kinda possible", but not practical.
If you remember your chemistry classes, you are also familiar with reversible reactions 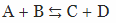 . During the early XIX century, it was actually postulated that all the reactions are reversible. Today, of course, we know that Berthollet was wrong.
. During the early XIX century, it was actually postulated that all the reactions are reversible. Today, of course, we know that Berthollet was wrong.
But there is something way more interesting, and those are oscillatory reactions
Mathematics behind the chemical oscillations
If you want to solve equations that describe chemical kinetics you need to solve differential equations.
And it's very easy to do this in Matlab, link 2 or even Matlab's Simulink if you are allergic to lines of code. And if you don't like anything related to Matlab, here is the code for Python.
Let's check the math, it's really simple:
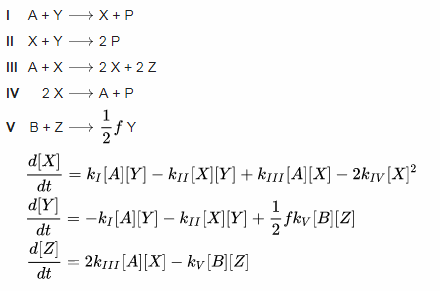
*I took this table from the Wiki page devoted to Oregonator model
Let's see how X is formed:
- in the first reaction, X is formed from A and Y, with the rate k1, so: [A][Y][k1]
- in the second reaction, X is used with the Y to form 2P, with the rate k2, so: -[X][Y][k2]
- in the third reaction, 2X are formed from A and X, with the rate k3, so: [A][X][k3]
- and finally, in the fourth reaction, 2X give A and P with the rate k4. Because we started with 2X, the equation is: -2[X]2[k4]
Analogous to this, you can write the equations for all other "ingredients".
And this core of the equations is responsible for oscillations.
Chemical Examples:
Belousov-Zhabotinsky reaction
After reading the math, you deserved a little movie break. The best part starts at 0:55.
How exactly does it work - we still don't know.
The reaction was initially discovered by Boris Belousov but initially, it was constantly rejected, because according to reviewer(S) this was thermodynamically impossible.
It took a whole decade before Anatol Zhabotinsky discovered an old, probably dusty, conference paper and continued the research.
The reaction itself was not that important in practical meaning, but it changed the way how we understand chemical reactions. In 1977, Nobel Prize was given to Ilya Prigogine for discovering that:
importation and dissipation of energy into chemical systems could reverse the maximization of entropy rule imposed by the second law of thermodynamics
<3 Check-mate creationists, although you fail even in the first step, by neglecting that the Second Law of Thermodynamics is applied in the examination of the closed systems. And the Earth is not closed. And the life could be still possible even if everything is closed :D <3
We still don't know what exactly is going on there.
Most papers describe the systems of 10 to 12 reactions, but the most complex (that I found...) is described with 80 reactions!
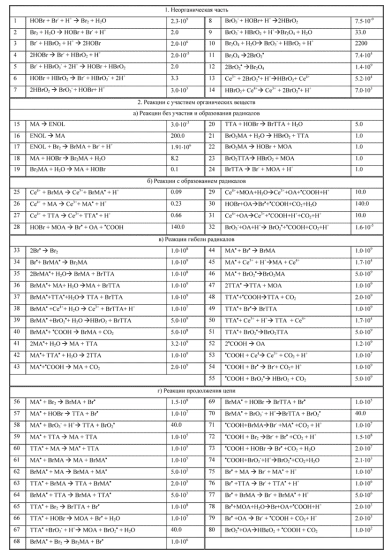
It sounds impossible, but this is PD image ("спасибо большое")
The biggest problem is that you can't sample it easily (*but maybe with the toy they have in Delft...), and you can't analyse it either without starting the reaction again.
What is done is the change in flow rates for the involved substances, using peristaltic pumps, but that's crude.
Bray–Liebhafsky Reaction
For the second reaction, we have the full lecture :
Important conclusions:
- It is possible to make very complex self-regulating and perpetuating systems even without the involvement of organic molecules
- It is possible to trick the second law of thermodynamics even in closed systems
- It is possible to obtain the attractor that can be the "timing belt" of the reaction "engine"
- There is a very simple mathematical background why those reactions are possible and not uncommon
- And finally, once you know this, the origin of life is maybe not that unusual nor impossible

Discover...

@alexs1320 : I think you are the first person in steemit with similar interests as that of mine. Although not a chemist, I am interested in oscillatory reactions which I was introduced during my PhD course work in systems biology. BZ reactions are very interesting and can be modelled using reaction-diffusion processes.
This is the true value of this network - to bring the people together and provide the healthy place where you can make the connections.
"Pro question": There is a strange idea about the *OH radical. According to some ideas, those are organized in "layers". Do you know some method that could simulate different ways how *OH "approach" to molecules?
I didnt get you. If I understand you correctly, are you asking if we can incorporate OH radical in MD simulations? Maybe QM based MD techniques can incorporate reactions. Not in classical MD.
Congratulations @alexs1320! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
To support your work, I also upvoted your post!
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP7000+ upvotes! Not bad, not bad...
Thermodynamics! A topic I've long forgotten... It's nice having to go back to it with this write up. This is nice. I love the calculations, the video( the first, I couldn't watch the second), and the fact that you highlighted the conclusion.
I must also say I saw something interesting before the 55 seconds you mentioned.. Lol, it's nice being here