Periodic History of Chemistry
The Periodic Table
The Periodic Table Of Elements
From the beginning of time, man has knowingly and unknowingly surfaced elements produced by the Earth. The very first elements that were uncovered by man with those of wealthy value such as gold and silver. These elements were used as a form of trade as well as power, since their discovery thousands of years ago. However, in contrast, certain elements were only discovered as recently as the 20th century due to the instruments that were created during those times. With the aid of these instruments, we were able to identify elements that were either radioactive, unstable or even elements that possessed both qualities. The majority of elements that we find today on the periodic table are present and are widely dispersed in nature in numerous forms of compounds. For decades that turned into centuries, scientists were unknown that these elements did exist in nature. It was thanks to the instrumental advances that took place in the 19th century that led to the discovery of many of these elements as, in the year 1800, only 31 known elements to man. By the year 1865, the number more than doubled to a phenomenal 63 known elements. In the proceeding years, this value of known elements gradually increased, and scientists realised that there were possibilities that a method of classification of these elements existed, but, unaware of how to do so. This was the birth of The Periodic Table.
The first breakthrough in the classification of elements took place in 1869, by the scientists, Dmitri Mendeleev of Russia and Lothar Meyer of Germany. These two scientists both noticed that a similar physical and chemical property of elements existed when these elements were placed in ascending order of atomic mass. We must understand however, that at this time of discovery, scientist were unaware of atomic numbers but these two scientists were able to identify that atomic mass increased in a specific order (today, we relate this to the atomic number of the element, which indicates that an increase in atomic mass generally increases the atomic number). The resulting table from the experiments conducted by these two scientists were the foundations of the modern-day periodic table.
As scientists, they were aware that in the proceeding years, many more discoveries of new elements would be found. By understanding this concept, they proceeded by using one of Mendeleevs procedures, in which the elements that portrayed similar characteristics should be grouped in the same family. In application of this procedure, many spaces were left available for those elements there were not discovered at the time, such as gallium and germanium (Ga and Ge, respectively). It must be noted though, due to the work and experiments performed by the scientists, they were able to predict elements that were not yet discovered by man by making reference to the observed patterns in the chemical compositions of the known elements. For example, Mendeleev predicted the existence as well as the chemical and physical properties of the two previous elements, gallium and germanium. The only exception was that the names of these elements were unknown so when naming them, they employed pseudo names for these elements, which are, eka-aluminium and eka-silicon respectively (which translate to ‘under’ aluminum and ‘under’ silicon, respectively). His predictions were of extremely high accuracy, with some properties having a minimal or no percentage error.
The following table will indicate the accuracy between the predictions for eka-silicon (chemical and physical properties), made by the duo of scientists, against the properties observed when germanium was discovered. An interesting point to note, is that the predictions made by the scientists were done in 1871, whereas the discovery of germanium only occurred in 1886.
| Physical And Chemical Properties | Predictions of Mendeleev | Properties upon Discovery |
|---|---|---|
| Atomic Mass (g/m-3) | 72 | 72.59 |
| Denisty (kg/m3) | 5.5 | 5.35 |
| Colour | dark gray | greyish white |
| Melting Point (degree celcius) | extremely high | 947 |
| Specific Gravity (J/kg.K) | 0.305 | 0.309 |
| Density of Oxide Formation (kg/m3) | 4.7 | 4.70 |
| Density of Chloride Formation (kg/m3) | a little under 100 | 84 |
Evolution and adaptation are processes that no one can hinder or prevent from occurring. Everything in life undergoes these processes and the development of the periodic table is no exception to this. In the years to follow from the development of the foundation of the periodic table, new discoveries in chemistry allowed for a more accurate depiction of elements. In 1913, an English born physicist by the name of Henry Moseley uncovered the concept of atomic numbers of elements. His theory stated correctly that the atomic number of an element is equal to the number of protons in the nucleus of the atom of that element as well as, in the case of a neutral element, was equivalent to the number of electrons in the orbitals as well.
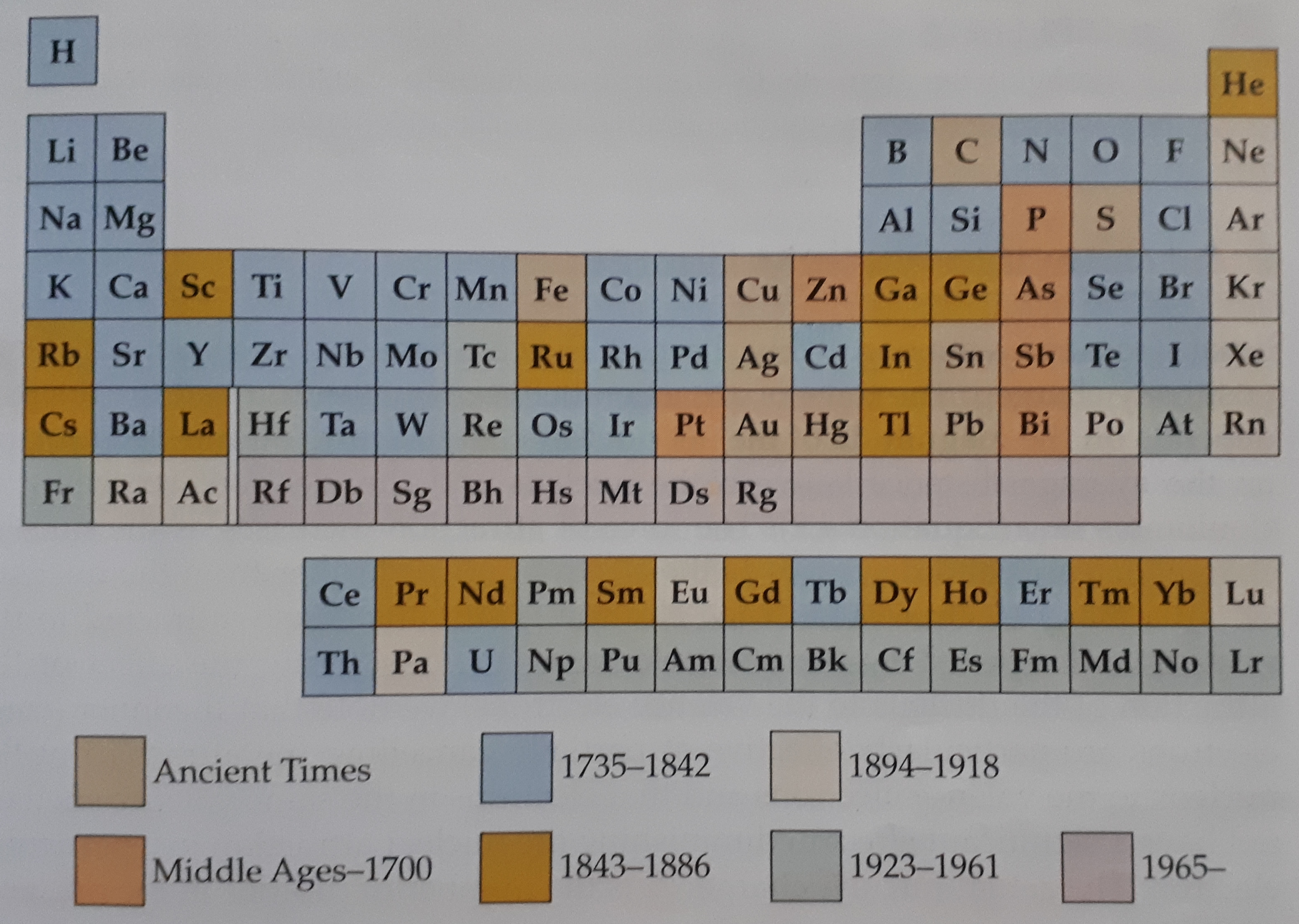
Discovery Eras of Elements
By the identification of the atomic number, the periodic table became more accurate and cleared up a few problems that were found in the foundation table. An important characteristic of the improved periodic table, is the use of atomic numbers rather than atomic masses for classification of elements. This method eradicated a lot of problems that occurred due to abnormalities in certain patterns of the elements. An example would be the following:
Let us consider Argon and Potassium. Argon has an atomic number of 18 while potassium has an atomic number of 19, but when considering the elements respective atomic masses, we find ourselves in a predicament, whereby, the atomic mass of Argon is greater than the atomic mass of Potassium, thus, when elements were based on the atomic numbers rather than the atomic masses, a constant increase in the atomic number was observed rather than an erratic pattern observed when atomic masses were considered. Moseleys' studies were also noted to identify the "holes" that were left in the periodic table by the previous scientists who attempted to identify and predict elements that were not yet discovered.
This brings us to the end of my post on the periodic table. As we can see, this table is a product of years of experimental data as well as human innovation and dedication to science and chemistry. We should be thankful to the hard work and effort put into the discovery of these elements as, had these elements not been found, our lives would be drastically different.
Electron Orbitals
Thank you Dimitri Mendeleev, thank you Lothar Meyer, thank you Henry Moseley, and to every other chemist out there through history for their contribution to the worlds most important table, on behalf of the worldwide science community.
Images are linked to their sources in their description
The End
References:
[1] https://en.wikipedia.org/wiki/Periodic_table
[2]https://www.livescience.com › Planet Earth
[3]https://chem.libretexts.org/Core/Inorganic.../Periodic_Trends_of.../Periodic_Trends
[4]https://en.wikipedia.org/wiki/Periodic_trends
[5]Chemistry,The central science: A broad perspective
[6]https://en.wikipedia.org/wiki/History_of_the_periodic_table


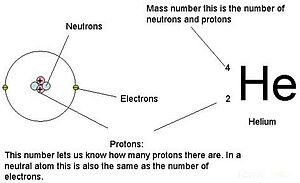
Immagine CC0 Creative Commons, si ringrazia @mrazura per il logo ITASTEM.
CLICK HERE AND VOTE FOR DAVINCI.WITNESS
Greetings from @davinci.witness and the itaSTEM team.