Photoelectrolysis of Water. Renewable energy [1,2,3]
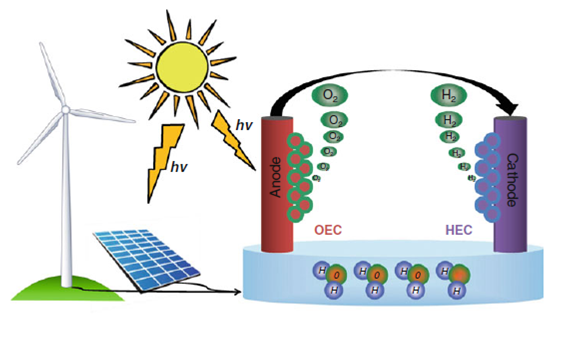
Schematic representation of the splitting of water via electrolysis, utilising electricity derived from renewable sources such as wind and solar, and photoelectrolysis, where the electrodes directly harvest the solar energy. Oxygen evolution catalysts (OEC) are located on the anode and hydrogen evolution catalysts (HEC) are located on the cathode. Adapted from Joya et al. (2013). (Book: Photoelectrochemical Solar Fuel Production From Basic Principles to Advanced Devices)
Currently there is a growing interest in the use of solar energy as an alternative energy for the production of clean and renewable energy, such as the production of hydrogen through, photocatalytic rupture or photoelectrochemistry of water, however for the materials studied so far , The catalytic activity remains low. Therefore it is urgent the development of new materials with a higher activity.
The photoelectrochemical decomposition of water requires in its simplest way to find absorbent species, which once excited, act simultaneously as a good reducer and oxidant, so that they can reduce water to H2 and oxidize it to O2. The process of the photoelectrochemical reaction for a semiconductor is considered as the absorption of light energy, with an energy equal to or greater than the separation between the valence band and the conduction band. In theory the decomposition of water can be carried out if the potential of the conduction band of the semiconductor is more negative than the potential of evolution of hydrogen and if the potential of the band of valence is more positive than that of the evolution of oxygen.
The liberation of oxygen in semiconductor electrodes was first observed in 1968 but it was not until 1971 when Fujishima and Honda [4,5] suggested that this effect could use light to decompose water into hydrogen and oxygen, using as photoanode the rutile of TiO2 And Pt as the secondary electrode for proton reduction; The simplest design of the electrochemical cell for photoelectrolysis of water consists of two electrodes immersed in an aqueous electrolyte electrically connected to a wire, where one of the electrodes is a metal and the other is a semiconductor, depending on the type of conductivity of the semiconductor We could have oxygen or hydrogen production (oxygen is released if the semiconductor is n-type, hydrogen if it is p-type). In order for the electrolysis of water to occur, the following criteria must be taken into account:
- The edges of the valence and conduction bands must overlap with the acceptor and donor levels of the water decomposition reaction. What makes it necessary that the separation between bands is 1.23 eV, which corresponds to the reversible potential of the decomposition reaction of the water.[6]
- The charge transfer on the surface of the semiconductor must be fast enough to avoid photocorrosion and the displacement of the edges of bands that results in the loss of photons of energy.
When conducting the excitation of a semiconductor, a hollow-electron pair is generated, resulting from intrinsic ionization on the conduction band.
Producing in this way the water split due to an increase of the photo voltage produced by the excess of charge minorities (hole).
In this way the following reactions occur.
Total reaction [5,6]
References
[1.] J. Nwotny, C. Sorrel, T. Bak. L.R. Sheppard, Solar-hydrogen: Unresolved problems in solid-state science Solar Energy, 2005, 78, 593-602.
[2.] V.M. Aroutiounian, V.M. Arakelyan, G.E. Shahnazaryan, Metal oxide hotoelectrodes for hydrogen generation using solar radiation-driven water splitting Solar Energy, 2005, 78, 581-592.
[3.] Zhigang Zoua, Jinhua Ye, Hironori Arakawa., Photocatalytic water splitting into H2 and/or O2 under UV and visible light irradiation with a semiconductor photocatalyst, International Journal of Hydrogen Energy, 2003, 28, 663 – 669.
[4.] Fujishima, A.; Honda, K. Electrochemical Photolysis of Water at a Semiconductor Electrode. Nature 1972, 238, 37–38.
[5.] Hashimoto, K.; Irie, H.; Fujishima, A. TiO2 Photocatalysis: A Historical Overview and Future Prospects. Jpn. J. Appl. Phys. 2005, 44, 8269–8285.
[6.] Harris, L. a; Wilson, R. H. Semiconductors for Photoelectrolysis. Annu. Rev. Mater. Sci. 1978, 8, 99–134.
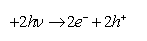
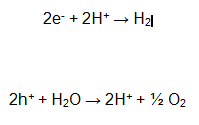
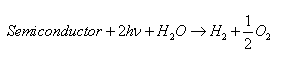
Interesting report for the use of renewable energies