making of sodium carbonate (Na2CO3 )oR soda ash
Is one of the most useful chemical substances of the lithium family.It has been known and used since very early times. Large deposits of this salt occur in Owens lake in california and other parts of the world .
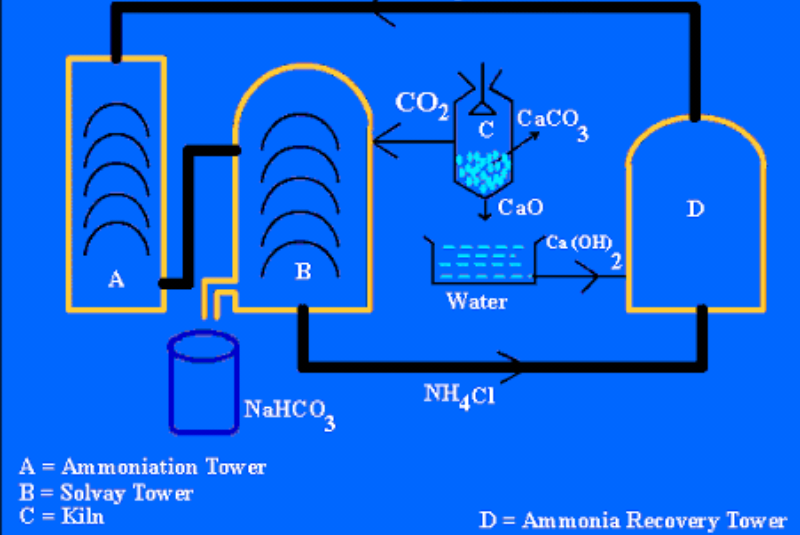
Ernest solvay , a belgian chemist ,devised "Ammonia solvay process " ,in 1861 to produce pure sodium carbonate
RAW MAERIAL :-
the raw materal required in this solvay process are :
Concentrated brine
limestone
ammonia
coal
The whole process is taken in three steps,
STEP 1:-
SATURATION OF BRINE WITH AMMONIA (NH3)
a saturated solution sodium carbonate chloride called brine is allowed flow down in ammoniacal toWer . The lower consist of mushroom baffles at short interval, which control and properly separated brine from ammonia
ststep 2
CARBONATION OF AMMONIATED BRINE
astream of co2 produced from heating lime stone is react with ammoniated brine in CARBONATION tower (solvay tower) to forMM sodium carbonate