making of caustic sode ( sodium hydroxide ) (NaOH)
Sodium hydroxide is a substance of great commercial importance and is, therefore, prepared on a large scale . It is corrosive and cuastic to touch and causes painful burns, that is why it is called caustic soda the name caustic soda was given bto the substance kn account of its power dissolving organic matter
PREPARATION:
sodium hydroxide is now manufacturer by electrolysis pprocess, known as castner-kellner's process
CONSTRUCTION OF CELL :-
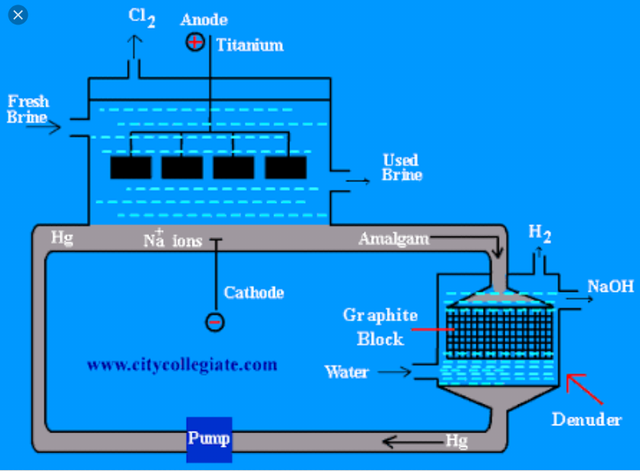
it consist of a large rectangular trough divided into compartments by slate partitions which do not touch the bottom of the cell. The bottom of cell is coverd with mercury and this is brought into circulation with the help of an ecentric wheel and a lower ciculer pipe like region. In the upper-operation, titanium plates hang vertically downward and acts as anode. Inthe lower region, mercury flows by mean of Hg pump and this stream of following mercury (Hg) acts as cathode. There is a borad region at the mid lowerr side of the cell called "Denuder" , whose function is to separated sodium from Na/Hg amalgam
CHEMISTRY OF CELL :
The electrotylte sodium chloride is introduced in the upper portion of the cell and electricity is passed . The negative ions (Cl OH) migrates towards the anode where they get oxidized . chlorine (Cl2) gas is libeated at the anode .
2Cl ---------------------------------- Cl2 + 2e
2OH ----------'---------------------- H2O + [O] +2e
Since H+ ions have a high over-voltage at mercury electrode, they are not discharged. Instead, Na ions are discharged at the Hg cathode and from Na/Hg amalgam
2Na + 2e --------------''--------------- @Na (reduction)
Na + Hg ------------------------------ Na/Hg (amalgam)
Mercury containing dissloved sodium is sent th another chamber called Denuder where sodium reacts with water , forming sodium hydroxide and hydrogen
2Na/Hg + 2h2O ---------------------- 2NsOH + H2 + Hg
Mercury is recycled to dissloved more sodium . The denuder is packed with graphite blocks as hydrogen is easily liberated over graphite surface