RENEWABLE ENERGY BIOETANOL FROM CASSAVA
One of the most promising alternative energy is bioethanol. Bioethanol is a biomass that is the main ingredient of ethanol from plants and generally uses a fermentation process. Ethanol or Ethyl Alcohol C₂H₅OH is a colorless, biodegradable, low toxicity liquid and does not cause great air pollution when leaking. Burning ethanol produces carbon dioxide (CO2) and water. Ethanol is a high-octane fuel that can replace timbale as an increase in octane grades in gasoline. By mixing ethanol with gasoline, it will oxygenate the fuel mixture so that it can burn more perfectly and reduce exhaust emissions (such as carbon monoxide).
Today, the world's energy needs are increasing as the supply of energy from fossil fuels that have been relied upon is limited. Therefore, an alternative energy source is needed to overcome the energy crisis. One alternative energy source being developed is bioethanol. Bioethanol can be produced by means of glucose fermentation using Saccharomyces cereviceae yeast.
Ethanol is widely used as fuel, antiseptic solvent, sterilizing agent, antifreeze and also used in alcoholic beverage industry. This proves that a lot of attention has now been shifted to alcoholic fermentation. Today many developed countries have turned their attention to some alternative energy sources other than oil, such as ethanol. The use of ethanol as an energy source is not new, because this technology has been tried in many countries. In addition, ethanol is also used in many ways and its needs will continue to increase in the future. One method for producing ethanol is by fermentation. A number of microbes such as yeasts, bacteria and fungi have the ability to produce ethanol from the genus Saccharomyces, Kluyveromyces, Candida, Schwanniomyces, Endomycopsis, Pichia, Fusarium, Rhizopus, Zymomonas, Clostridium, Thermoanae robium, and Thermobacteriodes, Aspergillus niger.
A potential source of bioethanol developed in Indonesia is cassava. Cassava is a plant that has been long known by Indonesian farmers, although not native to Indonesia. Cassava was first imported by the Dutch colonial government in the early 19th century from Latin America. Because it has been long known by Indonesian farmers, the development of cassava to be processed into bioethanol raw materials is not too difficult. Currently cassava is widely exported to the US and Europe in the form of tapioca. In the country, cassava is used as raw material for alcohol manufacturing industry. Tapioca starch is also used in the glue, chemical and textile industries. Indonesia is the fourth cassava producer in the world.
In the country, cassava is usually only used as animal feed and traditional food after rice and corn. Therefore, the price of cassava is very volatile and does not provide an adequate profit for the farmer. Development of bioethanol is expected to be a solution of renewable energy sources while increasing the income of cassava farmers. With this step, the price of cassava will be stable so as to provide sufficient profit. The problem of the future renewable energy crisis will be resolved and bring Indonesia into an energy independent country.
Formulation of the problem
a. What is bioethanol?
b. Why is "Cassava" selected as raw material for bioethanol manufacture?
c. What are the ingredients contained in cassava?
d. What are the benefits of bioethanol fuel compared to fossil fuels?
e. What are the tools used to make cassava bioethanol?
f. How is the process of making bioethanol made from raw cassava?
Aim
Knowing what is bioethanol.
Knowing potential materials that can be processed into bioethanol.
Knowing the content contained in cassava.
Knowing the process of cassava processing into bioethanol.
Analyzing the comparison of cassava yielded bioethanol
Knowing the advantages of cassava bioethanol compared to fossil fuels.
Bioethanol has been used by humans since prehistoric times as a drunkard in alcoholic beverages. The mixture of near-purity bioethanol was first discovered by a Muslim chemist who developed the distillation process at the time of the Abbasid Caliph with a famous researcher at the time. Since 1908 Ford model T cars already use bioethanol as fuel. However, in the 1920s fuel from petroleum that was cheaper to be dominant so that bioethanol received less attention. Recently, with rising prices of petroleum bioenatol again gained attention and has become an energy alternative that continues to be developed.
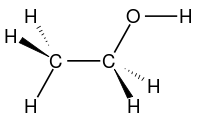
Bioethanol is often written with EtOH (ethylOH). The ethanol molecular formula is C2H5OH or the empirical formula C2H6O or its wake formula [CH3-CH2-OH]. Bioethanol is a group of metals (CH3-) strung on methylene (-CH2-) and stranded in the hydroxyl group (-OH).
Bioethanol is a liquid from the fermentation process of sugars that comes from carbohydrates using the help of microorganisms. Bioethanol can also be interpreted as chemicals produced from food containing starch, such as cassava, sweet potato, corn, and sago. Bioethanol is a fuel of oil that has properties such as premium oil.
The raw material for making bioethanol is divided into three groups:
a. Sucrose ingredients
The ingredients included in this group include foam nira, sugarcane, coconut palm, palm sugar, and cassava juice.
b. Heartbeat
Starch removal process by amylase enzyme. Materials belonging to this group are ingredients that contain starch or carbohydrates.
c. Cellulosic material (lignocellulose)
Cellulosic material (lognoselulose) means plant material containing cellulose or fiber, such as wood, straw, banana stem, and others.
Based on these three types of raw materials, cellulose material is a material that is rarely used and quite difficult to do. This is because the lignin is difficult to digest so that the process of glucose formation becomes more difficult.
The use of bioethanol as a fuel actually has long been known as previously mentioned that in 1908 Henry Ford made a quarcycle car and since 1908 Ford model T cars have been able to use bioethanol as its fuel. However, the use of bioethanol as biofuels was less desirable at the time, due to the presence of cheaper and abundant fuel. Currently, the supply of fuel oil continues to shrink plus the rising world fuel prices make bioethanol increasingly taken into account.
Bioethanol may be used in motor vehicles without altering the mechanics of the machine if it is mixed with gasoline with a bioethanol content of more than 99.5%. Comparison of bioethanol in Indonesia generally only adds 10% of total fuel. Blending 10% bioethanol absolute with 90% gasoline, often called Gasobol E-10. Gasobol stands for gasoline (gasoline) and bioethanol. Bioethanol absolute has an octane (ON) 117, while the premium is only 87.88. Gasobol E-10 proportionally has ON 92 or equivalent pertamax. In this composition, bioethanol is referred to as the most environmentally responsible octane additive and developed countries have shifted the use of Tetra Ethyl Lead (TEL) and Methyl Tertiary Buthyl Ether (MTBE).
Bioethanol can generally be used as industrial fuel for alcohol derivatives, the fuel mixture for Bioethanol Grade vehicles should be different according to their use. Bioethanol having 90% - 96.5% volume grade is used in industry, grade 96% - 99.5% is used in mixture for alcohol and pharmaceutical industry base. The amount of bioethanol grade used as fuel for vehicles must be completely dry and anhydrous so as not to cause corrosion, so bioethanol must have grade of 99.5% - 100%.
Bioethanol used as fuel has several advantages among them more environmentally friendly, because the fuel has an octane value of 92 higher than premium octane value 88, and pertamax value of octane 94. This causes bioethanol can replace the function of additives which are often added to enlarge octane values.
Additives are widely used such as tertiary butyl ether and Pb, but they are not very environmentally friendly and can be toxic. Bioethanol is also a fuel that does not accumulate carbon dioxide gas and is relatively compatible with gasoline-fueled engines. Another advantage of bioethanol is the simple way of making it fermentation using certain organisms.
Cassava, cassava, or cassava (Manihot utilissima) are the annual tropical and sub-tropical shrubs of the Euphorbiaceae tribe. Umbinya widely known as a staple food producer of carbohydrates and leaves as vegetables.
Cassava is a food crop and trade. As a trading crop, cassava produces dried cassava, cassava flour, ethanol, liquid sugar, sorbitol, MSG, aromatic flour, and pellets. As a food crop, cassava is a source of carbohydrates for about 500 million people in the world. Cassava is the largest producer of calories compared to other plants per day.
In addition, cassava is a plant that has good potential as bioethanol.
Systematic cassava plant / cassava is as follows:
Kingdom: Plantae
Division: Spermatophyta (seed plant)
Class: Dicotyledone (seeds in double pieces)
Odro: Eurphorbiules
Family: Eurphobiuceae
Genus: Manihot
Species: Manihot utilissima Pohl
Cassava as raw material of alternative energy source has carbohydrate content about 32-35% and starch content about 83.8% after it is processed into flour. Cassava plant as bioethanol feedstock can grow in less fertile land and its harvest period is not dependent on the season so that its harvest can last all year. Therefore, it is said that cassava is a potential raw material for making bioethanol.
Brazil is the starting center as well as the center of diversity of cassava. Cassava grows in areas with an average temperature of more than 18˚C with rainfall above 500mm / year. The productivity of cassava at farmer level is 14.3-18.8ton / ha.
Although data from the research center reported that its productivity could reach 30-40ton / ha. Cassava as a material of Fuel Grade Ethanol (FGE) suggested varieties that have properties: high starch content, high yield potential, and flexible in farming and harvesting. Cassava as bioethanol fuel has advantages that can grow on land less fertile, have high resistance to disease and can be regulated by harvest time.
The use of cassava as a raw material for bioethanol has been utilizing only the content of its patent, while other components such as cellulose and hemicellulose which also have the potential to produce bioethanol have not been fully utilized. This is due to the hydrolysis process using only enzymes capable of hydrolyzing starch fraction.
Ethanol superiority compared to gasoline
The continuous use of fossil fuels raises two serious threats: (1) economic factors in the form of guaranteed availability of fossil fuels for the coming decades, masala supply, and prices. (2) pollution from burning fossil fuels to the environment.
Countries that use ethanol will reduce their dependence on foreign oil imports, and also reduce the effects of unstable oil prices. Large domestic production of ethanol will ensure that money will continue to spin domestically instead of spending on expensive foreign oil. Of course an increase in domestic ethanol production will also create more jobs, and it is also likely to lower fuel prices.
The burning of ethanol is cleaner than fossil fuels which means reducing greenhouse gas emissions. This is the most significant ethanol advantage for the environment compared to fossil fuels.
Microorganisms on Fermentation
Alcohol can be produced from several fermented materials with the help of microorganisms, as a zimose-producing enzyme that catalyzes biochemical reactions to changes in organic substrate. Microorganisms that can be used for fermentation consist of yeast (yeast), yeast, fungi, and bacteria. These microorganisms have no chlorophyll, are unable to produce their food by fermentation, and use organic substrates for food.
Saccharomyces cereviseae is more commonly used to produce alcohol commercially than bacteria and fungi. This is because Saccharomyces cereviseae can produce large amounts of alcohol and have a tolerance to high alcohol levels. The resulting alcohol content is 8-20% under optimum conditions. Saccharomyces cereviseae that is stable, harmless or toxic, easy to obtain and even easy to maintain. Bacteria are not widely used to produce alcohol commercially, as most bacteria can not tolerate high levels of alcohol.
Processes in the Making of Bioethanol
- Likuifikasi
Liquification process is making the material become liquid, or melt the material. In this process additional ingredients are used alpha amylase enzyme. In this condition the flour will experience gelutinasi (thickened like jelly). At the optimum conditions alpha amylase enzyme works to break the structure of the flour chemically into complex sugars. Amylase is an enzyme that breaks down starch or glycogen in which these compounds are widely present in plant and animal products. Amylase can be divided into 3 groups of enzymes:
· Α-amylase is an enzyme that breaks starch randomly from the middle or inner part of the molecule.
· Β-amylase which breaks down the sugar units of the starch molecule.
· Glucoamylase is an enzyme that can separate glucose from the substrate reducing sugar terminal.
In this study, used α-amylase enzyme. The α-amylase enzyme is one of the starch-breaking enzymes. The α-amylase enzyme hydrolyzes alpha 1,4 glycoside bonds in both amylose and amylopectin at random. Due to the influence of its activity, the starch intermittently becomes dextrin with chains along the 6-10 units of glucose, maltose, and other longer bonds. Amylose hydrolysis by α-amylase occurs through two stages. The first stage is the degradation of amylose into maltose and maltriosa that occur randomly, very quickly and followed by a decrease in viscosity. The second stage is a relatively sluggish phase of degradation that is the formation of glucose and maltose as the end result, starting from the tip reducer in one.
The α-amylase enzyme obtained from microbes is generally stable at pH 5.5-8.0 and its optimum temperature varies depending on the source of the enzyme.
The use of α-amylase in the process of starch hydrolysis is often also called likuifikasi, because of the rapid decrease of viscosity, and the speed varies for various substrates. The α-amylase enzyme can be isolated from various sources of microorganisms such as Aspergilus oryzae, Aspergilus niger, Bacillus subtilis, and so on. Special α-amylase from Bacillus substilis, is the most important source of liquidity in industrial processes, because α-amylase from these microorganisms is able to react at high temperatures above the gelatinization temperature of starch granules. In starch hydrolysis, α-amylase produces dextrin which is the substrate for the advanced stage.
- Sacrification
The process of saccharification is the process of breaking down complex sugars into simple sugars with the help of enzymes that can separate glucose from the non-reducing sugar terminals of the substrate. Yeast can not directly ferment starch. Therefore, the required stage of saccharification, which changes the starch into maltose or glucose using enzymes or acids. By utilizing the starch decomposing enzyme from microorganisms, the conversion of starch to produce maltose and dextrin is not fermented due to enzymatic hydrolysis. The chemical composition of starch is amylose and amylopectin. Amylose is a polymer of glucose which is a straight chain and quantitatively amylose can be hydrolyzed to produce maltose, whereas amylopectin will only partially hydrolyze.
- Fermentation
The fermentation process is intended to convert glucose into ethanol (alcohol) by using yeast. Fermentation is an anaerobic saturated or partially anaerobic carbohydrate oxidation process. In a food fermentation process such as sodium chloride is useful to limit the growth of most other organisms. A foul fermentation is contaminated fermentation, whereas the normal fermentation is a change of carbohydrate to alcohol. Humans utilize Sacchamyces cereviceae to carry out fermentation, both in foods and in alcoholic beverages. This type of microbe is able to convert liquids containing sugar into alcohol and CO2 gas quickly and efficiently.
Saccharomyces cereviceae is the most widely used microbial in alcohol fermentation because it can produce high, resistant to high alcohol content, resistant to high sugar content and keep doing its activity at 4-32˚C temperature. the process of metabolism in Saccharomyces cereviceae is a series of directional reactions that take place on the cell. In this process occurs a series of reactions that are remodel a certain material and produce energy and a series of other reactions that are synthesizing certain compounds to produce energy. Saccharomyces cereviceae is not capable of directly fermenting macromolecules such as carbohydrates, but because they have secreted enzymes capable of breaking glucoside bonds that can be fermented into alcohols or acids.
Alcohol obtained in this process is an alcoholic bias with levels of 8 to 10 percent by volume. Meanwhile, if the fermentation is used raw sugar, ethanol can be made quickly. Making ethanol with sugar raw materials also has its own advantages that require a smaller fermentation tub. The ethanol produced by the fermentation process needs to be enhanced by cleaning it from unnecessary substances.
- Distillation
Distillation is a process of evaporation and condensation which is carried out to separate the mixture of two tau more liquids into its fractions based on the difference of the boiling point. In general, the separation of glucose fermentation results using a two-vapor liquid system comprising certain components that are readily mixed.
As known above, to purify bioethanol to fuel more than 95% to be used as fuel. Alcohols of fermentation having a purity of about 40% have to pass through the detilation process to separate the alcohol with water by considering the difference in the boiling point of the two materials which are then condensed back.
Ethanol levels can not reach levels above 18 to 21 percent because ethanol with these levels is toxic to yeast producing ethanol so distillation needs to be done.
Tools Used in Making Bioethanol
Function of tools used in the manufacture of bioethanol
Grinding machine, serves to smooth the raw materials, can be purchased in the store selling industrial equipment.
Cooker tank, serves to cook and stir raw materials before being put into heat exchanger, can be made from used drums.
Heat exchanger, serves to cool the raw material (during the process of saccharification) faster, can be made from stainless steel.
Fermentation tank, serves to produce ethanol content of 6-12%. Can be made from used drums or stainless steel tanks.
Evaporator, serves to evaporate the ethanol to be flowed to distillation. Made of stainless steel. To set the evaporator on this tool is installed thermostat (temperature control device).
Distillation device, serves to condense the vapor of ethanol into liquid ethanol. Can be made from used drums or stainless steel. A small spiral pipe (used for curving pliers) is made of copper.
Making Bioethanol from Cassava
- Raw Material Preparation
The raw material used is cassava. Cassava that has been peeled and cleaned destroyed to break the arrangement of flour to be able to interact with water well.
- Hydrolysis
Hydrolysis is the conversion stage of starch to glucose. In this stage there are two stages, namely: stage likuifikasi and stage of saccharification.
a. Likuifikasi
In the process of likuifikas, cassava raw materials mixed with water so that the porridge is estimated to contain 27-30 percent starch. Carbohydrate content of starch or starch in cassava raw material is converted into complex sugar using alpha amylase enzyme through heating process at 90˚C. in this condition the starch will undergo gelatination. At the optimum conditions alpha amylase enzymes work breaking the flour chemically into complex sugars. The liquefaction process is completed with the parameters in which the processed slurry becomes more fluid as a soup.
b. Sacrification
The saccharification stage is the stage of breaking down complex sugars into simple sugars performed on a tube in a series of equipment for bioethanol production. Sakarifikasi involves the following process:
Cooling the slurry until the optimum temperature of the saccharification enzyme works.
Setting the optimum pH of the enzyme.
Appropriate addition of glucoamylase enzymes.
Maintain pH and temperature in the temperature range 50-60 ˚C until the saccharification process is complete.
- Fermentation
turned into simple sugars with a sugar content ranging from 5 to 12%. The next step is to mix the yeast in the raw material liquid and keep it in a sealed container at an optimum temperature range of 27-32 ° C for a period of 5 to 7 days (anaerobic fermentation). The whole process requires precision so that raw materials are not contaminated by other microbes. In other words, from preparation of liquification, saccharification, to fermentation must be in a contaminant free condition. During the fermentation process will produce liquid ethanol and CO2.
The result of fermentation is a liquid containing low alcohol of 7-10%. At 10% yeast ethanol levels become inactive due to excess alcohol which will result to toxicity to the yeast itself and shut down its activity.
- Distillation
Alcohol-fermented products with low levels are called birds, so they need to be increased in concentration by distilled stratified roads. Beer contains 8-10% alcohol. The purpose of this distillation process is to separate the ethanol from the water ethanol mixture. For a solution consisting of components of different boiling temperatures, and distillation is the easiest way to operate and also a thermally efficient separation method. At atmospheric pressure, water boils at 100˚C and boiling ethanol at a temperature of about 77˚C. it is this boiling point difference that allows the separation of water ethanol mixtures.
- Dehydration
The distillation product of 95% ethanol is not soluble in gasoline. For fuel subsidies ethanol required levels of 99.6-99.8% or called dry ethanol. For 95% ethanol purification required dehydration process using several ways, among others: 1. Chemical way by using limestone. 2. The way physics is pursued through the process of absorption using Synthetic Zeolt. The dehydration result is ethano 99.6-99.8% so it can be categorized as Fuel Grade Ethanol (FGE) which can then be used as standard pertamina feminine motor fuel. The tool used in this process is a dehydrator.
Steps in Making Bioethanol
In making bioethanol from cassava, the steps taken are:
Cassava as raw material peeled before and milled so that its size decreases.
Cassava goes into the cooking stage that is likuifikasi. Raw materials plus water, heated at 90-95˚C. During heating plus alpha amylase enzymes that work break down the structure of the flour chemically into complex sugars. In this condition the material will experience gelatinasi (thickened like jelly). This process is completed by being marked as liquid as steam.
Sakarifikasi, after cooled from liquification to 60˚C, then added enzyme glukoamilase which function to break up complex molecule become simple.
Then the fermentation stage, to convert sugar into ethanol and CO2. Fermenentation is done by mixing the yeast in the liquid of the raw material and keeping it in a sealed container at the optimum temperature range.
Then enter at the separation stage, distillation to separate ethanol in the fermented liquid. In the distillation process, at a temperature of 78 degrees Celsius (equivalent to the boiling point of alcohol) ethanol will evaporate earlier than water. The ethanol vapor in the distillator will flow to the condenser condenser so it becomes condensed into ethanol. Then, the ethanol content of 10%.
Dehydration, this stage is done so that the water content in the product is reduced. This stage can be done with a synthetic zeolt catalyst. Zeolt is a mineral that has very small pores, and can absorb water. And ethanol content obtained after going through this stage amounted to 99.7%.
Side Results Bioethanol Processing
The end of the ethanol distillation process produces solid and liquid waste. To separate the effect on environmental pollution, the solid waste by a certain process is converted into potassium fertilizer, biogas making materials, compost, basic ingredients of mosquito coil and animal feed. While the liquid waste is processed into liquid fertilizer. Thus bioethanol producers do not have to worry about related issues.
Conclusion
Bioethanol from sugary or starchy materials such as cassava or cassava, sugar cane, sap, sorghum, sweet potatoes and others.
How to make bioethanol with raw material milling process, liquification, saccharification, fermentation, distillation and dehydration.
Suggestion
With the abundance of cassava production in Indonesia, bioethanol is a highly probable source of renewable energy. In addition to reducing the country's dependence on fossil fuels that are increasingly diminishing in number, bioethanol fuel can increase farmers' income which will have an impact on the increasing economy of the people. The impact of bioethanol is environmentally friendly, so the government is expected to be more serious to be able to make bioethanol as a substitute fuel in Indonesia and the world.