What Makes Influenza So Deadly?
The influenza strains of virus are a member of the orthomyoxoviridae family, and are responsible for local epidemics and worldwide pandemics, about 12 pandemics have occured in the last 400 years due to this virus.
Influenza structure
There are three main types of influenza virus: A, B and C, and are mainly differentiated by their serological differences in their nucleocapsids (the part that surrounds genetic material). Within these main groups, there exists many subtypes, which differ in their surface proteins (neuraminidase (NA) and hemagglutinin (HA)). A feature not exclusive to influenza, but still an evasive one, is the viruses' outer envelope is derived from infected host cells after assembling and releasing from the host. This effectively gives new virions a camouflage against host-cell defense systems. The only defense against influenza is the hosts' ability to recognize two surface proteins present on the virial membrane: hemagglutinin (HA) and neuraminidase (NA).
The surface proteins: HEMAGGLUTININ (HA)
Hemagglutinin is the second main evelope protein of the influenza virus, it is glycosylated (as in it contains sugar groups) and functions as a virus-to-host hook, binding to the target membrane. HA recognizes groups on the hosts' own glycoproteins, called sialic acids. As mentioned before, HA plays a central role in viral infection and the immune response by the host. Additionally, hemaglutinin has the ability to make red blood cells clump together (agglutinate), in vitro.All in all, there are 18 known subtypes of HA, each encoded in their own virial plasmid segment: segment number 4. The strains that infect humans only contain three of the thirteen known HA subtypes, these are usually H1, H2 and H3. This molecule is synthesised at the rough endoplasmic reticulum , and then co-translationally inserted into the membrane. It is only cleaved after translation occurs, which produces two chains of HA1 and HA2, held together by several disulfide bonds. Below is a cartoon-structure of a HA monomer (it exists as a trimer; three monomers), obtained from microbe wiki:As you can see, the difference between the active form and native form is quite significant - and it's all caused by the loss of one proton. This massive conformational change allows HA to act as a membrane fusogen ( a fancy word that means that the virus can effectively fuse with the hosts' membrane), this occurs because virial attactment to the host makes viral particles to be taken up via endocytosis. This transport of virus particles lowers the pH inside the host cell and induces the hemaglutinin molecules to rapidly change shape, fusing the membranes together and infecting the host cell with its RNA. The rapid conformational change of the protein is irreversible, and involves binding to host HA receptor sites.
Neuraminidase, in brief
This relatively small protein is made of approximately 470 amino acids and four main domains:
- a cytoplasmic "head";
- a transmembrane "head";
- and a type of "stem."
As with HA, it contains disulfide bonds and is a glycosylated outer membrane protein, used to break down several host membrane strucutres, such as sialic acids. It also is involved in the membrane fusion action of HA and also assists in budding of new viral particles.
Infection by influenza
Since I'm pressed for time, I'll leave this image and another link below:Check this out (it goes into infection ins quite a bit of detail):
https://www.rapidreferenceinfluenza.com/chapter/B978-0-7234-3433-7.50009-8/aim/virus-replication
How the vaccines work, and why they don't last for this virus
Without the harmful section of the HA and NA proteins, you defense systems are able to recognise the new variations on the influenza virus, and hence is better equipped to destroy any infectious verisons of the viruses of the same type/subtype. The problem lies in the fact that influenza is highly variable and can not only mutate quickly to produce new types of subtypes, but it is subject to antigenic shift and antigenic drift.Antigen drift is where:
- The external glycoproteins, HA and NA change via point mutations in their sequences.
- It is a type of change which occurs annually and is reponsible for local epidemics
- Changes in the viral subtype antigenecity and the fact that the host has a partial memory on subtypes (your defensive cells eventually fail to recognise the same subtypes combinations)
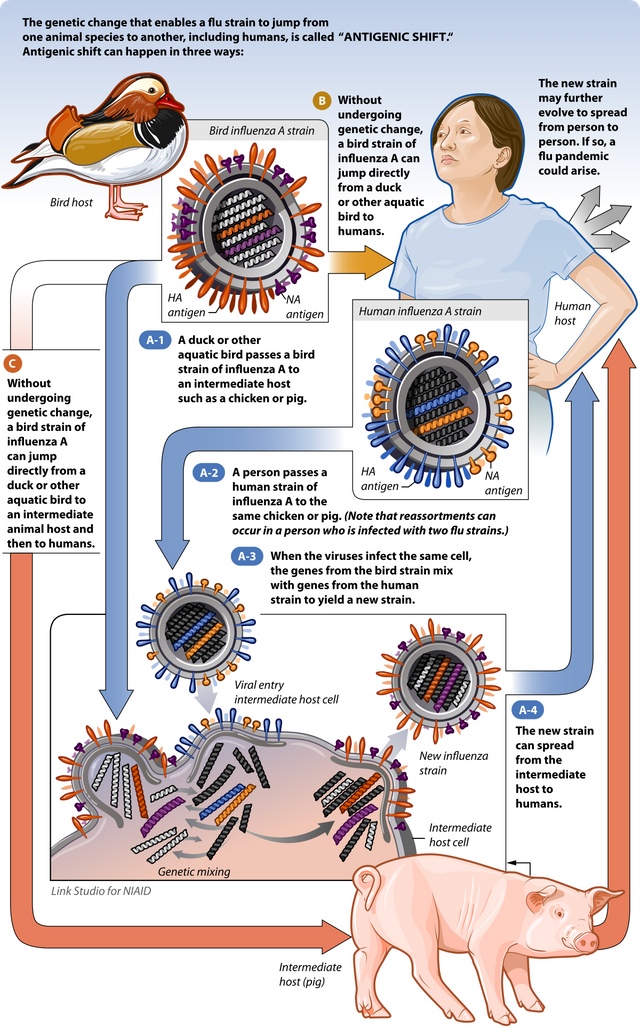
Antigen shift is where:
- the influenza virus can aqcuire a totally new form of HA in the human viruses, caused by two different types of influenza infecting a single cell (usually avian or swine) and forming a recombinant (mixed) virion. Thus, the virus' external structure and genes are completely different, and the host has a harder type recognising the new subtype of influenza.
- This causes a pandemic, which is a worldwide outbreak, and can cause a significant amount of deaths.
References
- Branden & Tooze, Introduction to Protein Structure (2nd Ed)., pages 79-84.
You can also find me on minds.com:
https://www.minds.com/MrElement
Congratulations @mr-element! You have completed some achievement on Steemit and have been rewarded with new badge(s) :
Click on any badge to view your own Board of Honor on SteemitBoard.
For more information about SteemitBoard, click here
If you no longer want to receive notifications, reply to this comment with the word
STOP