On the Regulation of the Human Energy Metabolism #1
In the first article on this topic, I'll start with exemplary organ-specific and load-dependent characteristics and needs of the very much fine tuned
Human Energy Metabolism

Image Source
All organs of an animal body work in concert to maintain a normal functioning. This requires metabolic specialization by some organs and cooperation with regard to the maintenance of a sufficient energy supply. Here three selected organs - that differ widely in their energy requirements - shall be discussed: the brain, skeletal muscles and the liver.
The brain makes up roughly 2 % of the body mass, but is responisble for as much as 20 % of the consumed energy in a resting human being. This high energy requirement is due to the plasma membrane Sodium-Potassium-ATPase, which maintains the membrane potential necessary for nerve impulse transmission. - For some general insight into neurotransmission have a look at another article of mine.
The brain's major metabolic fuel is glucose. Since the brain does not store glycogen, it requires a high glucose blood concentration of 5 mM glucose, with a level of below 2.5 mM being critical! During an extended fast of several days duration, the brain switches to the use of keton bodies as an alternative source of energy. This switch occurs long after all the body's glycogen has been used up. Hence gluconeogenesis from amino acids is required in the intermittent period.
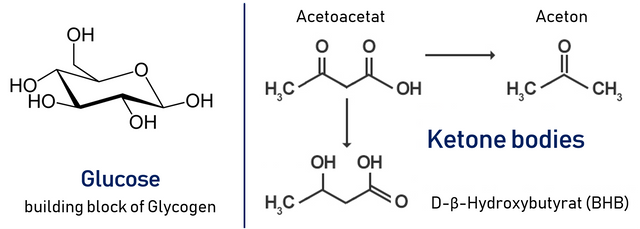
Our muscles use glucose, fatty acids and ketone bodies as fuel. Muscles lack the enzymes necessary for gluconeogenesis, hence they depend entirely on the supply of glucose through the bloodstream. Resting skeletal muscles use 30 % of the consumed oxygen. In response to a heavy workload, the metabolic rate of the muscle may increase 25-fold!
Initially, the muscle uses its phosphocreatine store to generate ATP for hydrolysis (which drives muscle contraction). After this initial period, of maximal 4 sec (!), the muscle shifts to ATP production via glycolysis of glycose-6-phosphate. A well-fed muscle consists of 1-2 % glycogen. The advantage of using glucose is, that it can be metabolized anaerobically, whereas fatty acids cannot.

The livers is the body's central metabolic organ controlling blood levels of all major fuels like glucose, fatty acids and ketone bodies. One of its major functions is to respond to insulin and other metabolic hormones and thereby control blood glucose levels.
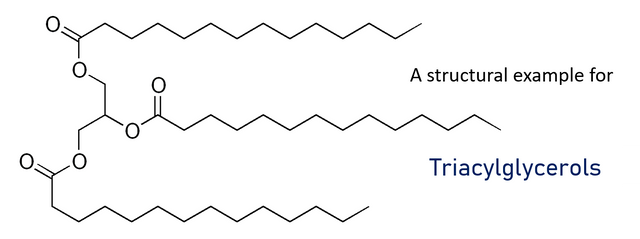
The prime source of energy for the liver is oxidation of fatty acids. Liver tissue cannot use ketone bodies as fuel. Whenever the demand of metabolic fuel is low, the liver produces triacylglycerols which are excreted as Very Low Density Lipoproteins (short VLDL) into the bloodstream and furthermore taken up by adipocytes in adipose tissue.
Now that we have established some metabolic characteristics of distinct organs, we will turn to the earlier already mentioned necessity of cooperation and interworking of these organs:
Let's have a look at the two most important cycles, that require an exchange of key metabolites between the liver and the skeletal muscles:
- The Cori Cycle
- The Glucose-Alanine Cycle
ATP required to power skeletal muscles is produced through oxidative phosporylation or, when ATP demand exceeds oxidative flux, by catabolism of glucose to lactate. Lactate is then transferred via the bloodstream to the liver where it is reconverted to pyruvate and used for gluconeogenesis. Thus, liver and muscles are linked by the bloodstream in a metabolic cycle, named after Carl and Gerty Cori, who first described it. A summarizing illustration is shown next:
The purpose of the Cori Cycle is to utilize liver ATP to generate glucose which is then used to produce ATP in the muscle tissue. The amount of lactate released during strenuous physical activity depends on the individual fitness.
Next up, the Glucose-Alanine Cycle: It is a similar cycle, that usually kicks in when the body is in "Survival Mode". It uses alanine rather than lactate, that travels from the muscle to the liver.
Some aminotransferases utilize pyruvate rather than a-ketoglutarate or oxaloacetate as α-keto acid substrate for transamination. The produced alanine is transported to the liver where it is transaminated to pyruvate which is then used to generate glucose via gluconeogenesis again using liver ATP.
During fasting or starvation, the normal cycle is broken as not enough glucose is available, which in essence means that muscle proteins themselves are used as the fuel.
In the liver, the amino group is used to form urea. Thus, the Glucose-Alanine Cycle is a net transfer of nitrogen from muscle tissue to the liver and physical activity leads to excessive formation of urea which is excreted not only via the kidneys but also through sweat glands.
Follow me to get more insight into this and many more scientific topics. :)
mountain.phil28
References:
- L. Rui, Compr Physiol., 2014, 4(1): 177-97
- M. Hargreaves, Clin Exp Pharmacol Physiol., 2000, 27(3): 225-8
- A. Falkowska et al. Int. J. Mol. Sci., 2015, 16(11): 25959–25981.
- T. Hlubin, Glucose-Alanine Cycle VS Cori Cycle
- Further information and non-direct-cited images are taken from
"Molecular Physiology" lectures at the TU Graz.
his beautiful postings, dropped in my account and upvote
Thank you very much! :)
Nice to hear, that there are people appreciating my work!
Best,
mountain.phil28
Yes, upvote mei
Beautiful post :) so nice keep going :)