Hydrogels and Usage Areas
Polymers are compounds with long chain and high molecular weight which result in the formation of more or less identical or different atomic groups more or less regularly with chemical bonds. If the polymer consisting of identical monomer units it is called as homopolymer, and when two different types of monomers are joined in the same polymer chain, the polymer is called a copolymer. Basicaly, hydrogels consist of hydrophilic copolymers or homopolymers.
Hydrogels are polymers that contain a large number of hydrophilic groups, which do not dissolve when left in aqueous media, and exhibit swelling properties by retaining a large amount of water. They are also called hydrophilic polymers because of their water-like properties. Hydrogels have functional groups such as -SO3H, -COOH, -CONH2, -OH and -NH2 which provide hydrophilic character to the polymer chains in their networks.
Due to these groups the cross-linked polymer starts to swell due to the increased water volume and mass due to the water that is bound. The water loving groups in the crosslinked polymer causes more swelling. Hydrogels with a high water holding capacity in the constructions also show very similarities to living tissues because they are in a soft and flexible structure.Scientists designed a new material for use in ophthalmology, and from that they were first hydrogel synthesized with HEMA (2-hydroxyethyl methacrylate) and EDMA (ethylene dimethacrylate) copolymerization in the early 1950s. The first hydrogel based soft contact lenses have been prepared these years. Studies on the biocompatibility of hydrogels were carried out in 1960s. Hydrogel synthesis was started for medical applications and it was also used to remove postoperative scars. Hydrogels have taken an important place in our lives with the work done in different fields in the years that have passed.
Hydrogel Preparation
The synthesis of hydrogels is accomplished by chemical initiator free radical polymerisation or radical chain polymerisation initiated by high energy beams. Hydrogel preparation by chemical cross linking occurs by direct cross linking using a cross linker in a small amount of one or more of the monomers. Chemical preparation of hydrogels consists of four steps. These are initiation, chain growth and ending with cross linking, merger or division. First, monomers that are chemically bonded to each other in the presence of crosslinkers form polymers, and then hydrogels are formed by transferring some of the monomers to the crosslinker and polymer chains to each other.
Excitation is effected by accelerated particles such as alpha, beta and gamma rays, electrons, protons and neutrons, and is similar in properties to photochemical polymerization at the radical chain polymerisation initiated by high energy beams. The advantages of this process are that the polymer can be made in solid, liquid, gas phases at the desired phase and the polymerization of monomers which are difficult to polymerize by other methods. Furthermore, the lack of a cross linker that limits the use of hydrogels in the formulation due to toxicity in food, pharmaceutical and pharmaceutical industries is a significant advantage of this method.
Although polymeric hydrogels can be prepared by a variety of techniques as I mentioned above, the most common method used is free radical cross linking copolymerization of monomers such as nonionic acrylamide (AAm) in the hydrophilic structure in the cross linker association N-N'- ethylenebisacrylamide (BIS). Ionic comonomers can also be added to the reaction mixture to enhance swelling capacity. Since the monomers used to prepare the hydrogels are usually solid at the polymerization temperature, the polymerization reactions require carried out in aqueous solutions. The hydrogel structure and properties depend on the cross-linker concentration, the concentration of the monomers and the conditions under which the hydrogel is directly formed, such as the chemistry of the building blocks.

Hydrogels can be classified in various species within themselves.
According to the preparation method;
- Homopolymer hydrogels
- Copolymer hydrogels
- Multiple polymer hydrogels
- IPN (interpenetrating networks) hydrogels
According to the side groups they have;
- Neutral (non-ionic) hydrogels
- Ionic hydrogels
According to physical structures;
- Amorphous hydrogels
- Semi crystalline hydrogels
- Hydrogen bonded hydrogels
According to the state of crosslinking;
- Physical cross linked hydrogels
- Chemical cross linked hydrogels
According to sources;
- Natural hydrogels
- Synthetic hydrogels
According to the water content;
- Low swelling grade (20-50%) hydrogels
- Medium swelling grade (50-90%) hydrogels
- High swelling grade (90-99.5%) hydrogels
- Superabsorbent (> 99.5%) hydrogels
According to chemical stability;
- Biodegradable hydrogels
- Non-biodegradable hydrogels
Usage Areas
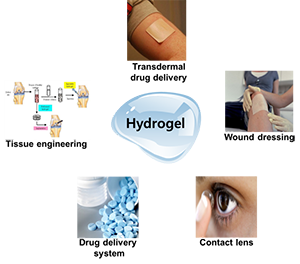
Depending on these classifications, the usage areas of hydrogels also vary. For example, hydrogels exhibit swelling or shrinking behavior in response to environmental variables such as pH, temperature, ionic strength and electric field. These properties make it possible to use them as artificial sensors in biomedical fields. The fact that the volumes of the hydrogels change too much with little change of the external influences cause them to become a material used in technology. Rubbery texture and excellent biocompatibility resembling living tissues make hydrogels attractive to many areas. Hydrogels are devices that are diagnosed, treated and implanted in the biomedical field, are used as superabsorbent polymers in wastewater cleaning with the capture of heavy metal ions and organic pollutants in the environment area. Hydrogels are also used extensively in fields such as biotechnology, bioengineering, pharmacy, agriculture, veterinary, food industry, telecommunication.
Hydrogels in general used in;
- Controlled release systems,
- Artificial organ construction
- Contact lens
- Enzyme arrest systems
- Biosensor
- Cosmetics sector
- As an additive in the food sector
- Artificial cornea
- Magnetic separation
- Bone disease treatment
- In many applications such as synthetic cartilage and the like
- Water purification
- Heavy metal dyestuff removal
- Ion exchange applications
- It is also used effectively in areas such as controlled release of fertilizers and pesticides
| Usage Areas | Hydrogels |
|---|---|
| Wound cover | Polyurethane, polyethylene glycol, polypropylene glycol, polyvinyl pyrrolidone, methyl cellulose, carboxymethyl, cellulose, alginate |
| Drug transport and pharmaceutical | Polyvinyl pyrrolidone, starch, polyacrylic acid, carboxymethyl cellulose, polyvinyl alcohol, acrylic acid, methacrylic acid, chitosan |
| Dental material | Polyvinyl alcohol, polyacrylic acid, hyaluronan, collagen |
| Injectable polymer system | Polyesters, polypeptides, chitosan |
| Technical products (cosmetics, pharmaceuticals) | Arabian gum, pectin, chitin, chitosan, heparin, starch, alginate |
| Others (Agriculture, waste treatment, separation, etc.) | The starch, polyvinyl alcohol, poly (N-isopropyl acrylamide), polyvinylmethyl ether |
As you can see, hydrogels have many uses and applications. But of course it is not limited to these, there are many tried and still testing areas. I believe that there will be many different places in the technological sense with the works done.
References
- Garner, C.M., The synthesis of a super absorbent polymer, Modular Laboratory Program in Chemistry, 739, Baylor University, 2000
- Nichifor, M., Zhu, X. X., Copolymers of N-alkylacrylamides and styrene as new thermosensitive materials, Polymer, 44 (10), 3053-3060, 2003
- https://www2.chemistry.msu.edu/faculty/reusch/virttxtjml/polymers.htm
- https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4348459/figure/f0080/

Hello friend, i think this is perfect post. Thank you for your information.
img credz: pixabay.com
Nice, you got a 27.0% @trafalgar upgoat, thanks to @kedi
It consists of $9.42 vote and $3.14 curation
Want a boost? Minnowbooster's got your back!