ARTIFICIAL EMBRYOS

In the early stages of pregnancy, the developments that influence everything from whether the embryo will implant in the possible diseases of the child later in life, are hidden from the view of the researchers. Now, a new study offers a window into that black box.
Researchers have found a way to grow artificial mouse blastocysts, an early embryonic structure, in the laboratory by combining stem cells in a dish. Artificial embryos, called blastoids, resemble natural ones so closely that when they are transferred to the uterus of a mouse, they are implanted and begin pregnancy.
Because they can be easily produced in large quantities, blastoids can serve as new models for drug development, which hopefully leads to treatments for infertility and early interventions for adult diseases.

A blastocyst is an early mammalian embryo, before it is implanted in the uterus. It is a hollow sphere formed by less than one hundred cells and consists of an outer layer of cells, the future placenta and a small group of internal cells, the future embryo. Therefore, it contains all the stem cells that will form the entire embryo and the entire placenta.
Other laboratories have previously shown that the stem cell lines representing these internal and external parts can be grown independently and multiplied in the laboratory. By combining these mouse stem cells, we have now succeeded in creating embryo structures in the laboratory. Artificial embryos are so similar to natural embryos that they successfully nest in the womb and begin a pregnancy.
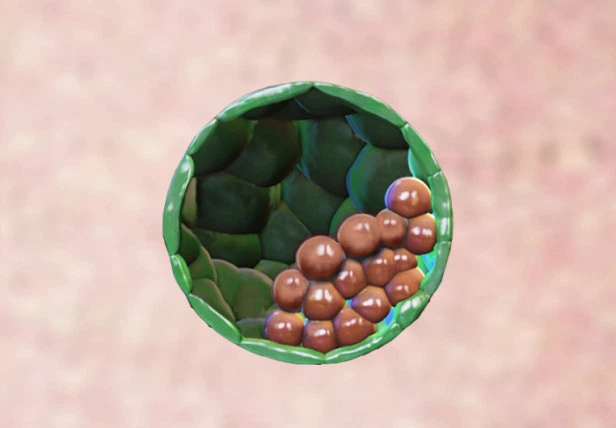
A representation of a blastoid, which is a synthetic embryo formed in the laboratory, from stem cells. The green cells are the stem cells of the trophoblast (the future placenta), while the red cells are the embryonic stem cells (the future embryo).
This completely new method allows us to understand the hidden processes at the beginning of life, find solutions for fertility problems and develop new drugs without research in laboratory animals. At present, very little is known about the development of stem cells during that first period of pregnancy. Early embryos are not only tiny, the diameter of a hair, but they are also practically inaccessible in the uterus. These artificial embryos can be formed in large quantities and studied in detail, allowing researchers to understand deeply the process of early embryonic development. This knowledge is of vital importance because the small anomalies at the beginning of pregnancy can have important consequences: they can prevent the implantation of the embryo or can contribute to the development of diseases much later in life.

To promote self-organization of stem cells, we first cultured embryonic and trophoblastic stem cells from mice independently in the laboratory. Embryonic stem cells can form the entire embryo, and trophoblast stem cells can form the entire placenta. By combining them in a specific proportion and stimulating them with a cocktail of molecules, we initiate their communication and encourage them to self-organize. This creates a structure similar to an embryo that closely resembles a natural early embryo.
For the time being, we know that they can be implanted in the uterus, multiply and differentiate to produce types of cells that are known to appear early during pregnancy. For example, trophoblast cells attract the mother's blood vessels, fuse with them, and establish the first connection that irrigates the embryo with the mother's blood. However, we do not form a complete mouse at this time. We are currently looking for research ways to understand these initial processes and further stimulate the development
The formation of embryo models after implantation, known as Gastruloides, in recent years was an advance started by Susanne van den Brink and Alfonso Martínez Arias. Sarah Harrison and Magdalena Zernicka-Goetz also did a beautiful job adding a type of extra embryonic trophoblastic tissue. These model embryos mimic a stage in which the embryo has already been implanted. They do not form all the embryonic tissues. For example, they lacked the tissues that mediate the union to the uterus (trophectoderm) and those that later support the embryo.
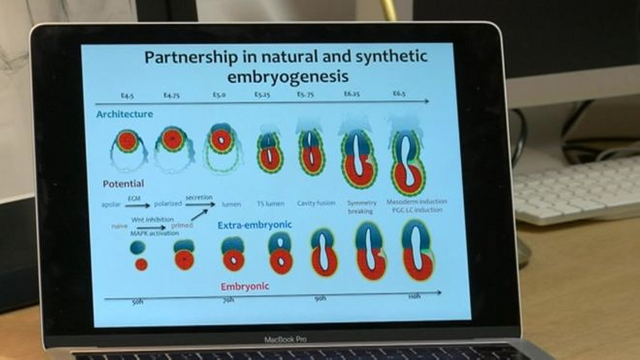
What we are forming are very early preimplantation embryos that include these extra-embryonic tissues, such as the trofectoderm that promotes attachment to the uterine wall of the mother. The blastoid is, therefore, an embryo model at an earlier stage that formed the tissues necessary to implant in the uterus.
Because they are made with stem cells that can be multiplied and modified (genetically) in the laboratory, blastoids can be manufactured in large quantities and studied in great detail. Blastoids, therefore, make it possible to run genetic or drug tests, and generate enough material for deep genetic analysis (epi).
Lately, epidemiologists have discovered that very small defects that occur at this early stage of development have enormous and persistent consequences. They can prevent the blastocyst from implanting or leading to a suboptimal development of the placenta and the fetus, which increases the occurrence of chronic diseases (eg, cardiovascular diseases) later in life. Establishing a perfect path for the development of the embryo has very important and long-term consequences, and is a great lever to prevent global health problems. For the first time, we can study these phenomena in great detail and perform drug tests to find medicines that can prevent infertility, find better contraceptives or limit the appearance of epigenetic marks that appear in the blastocyst and cause diseases during adulthood.
SOURCE:
Thank you in advance for your valuable time and for having read this publication. I hope it has been to your liking, soon I will make other publications of interest. Do not forget to leave a comment, criticism or constructive contributions to this topic and if you liked give me upvoto favorable. Greetings Steemists. Thanks for your attention........



artificial intelligence has really help science in all ratification of life now coming to artificial embryos
If it is already a fact that it can be done with artificial embryos