Regenerative Medicine Market Stand Out as the Biggest Contributor to Global Growth and Will Hit 26.1% CAGR By 2026
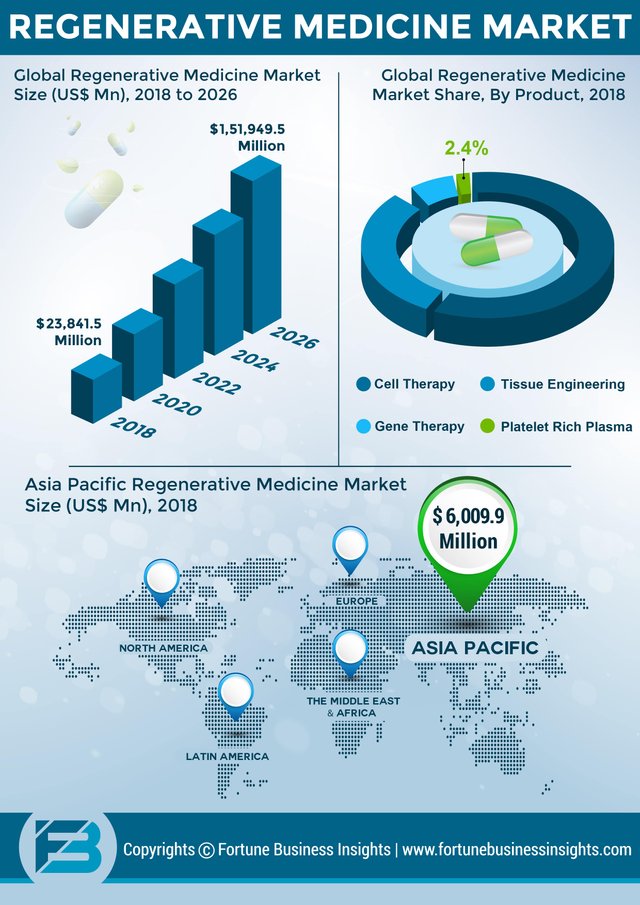
The global regenerative medicine market is likely to expand considerably in the coming years due to growing applications in the treatment of chronic diseases. Fortune Business Insights states that the market will reach US$ 151,949.5 Mn by the end of 2026, thereby exhibiting a CAGR of 26.1%.
Recent developments in treatment of acute and chronic diseases can be attributed to advances in regenerative medicines. Regenerative medicine mainly focus on identifying the root cause of the disease and aims at locating, repairing, and regenerating the non-functioning body cells. As most of the chronic and acute diseases are inclined towards the elderly and geriatric population, the growing geriatric population serves growth of the global regenerative medicine market in the coming years. The ability of regenerative medicine to reduce the burden for some of the most severe chronic diseases will create a high demand for the products in the coming years.
Browse Complete Report Details@ https://www.fortunebusinessinsights.com/industry-reports/regenerative-medicine-market-100970
KEY INDUSTRY DEVELOPMENTS:
-In 2018, Novartis received EU approval for one-time gene therapy Luxturna, which has been developed to restore vision in people with rare and genetically-associated retinal disease.
-In 2018, Novartis received EU approval for its CAR-T cell therapy, Kymriah.
-In 2017, Integra LifeSciences launched its product, Integra Dermal Regeneration Template Single Layer "Thin" for dermal repair defects reconstruction in a one-step procedure.
Novartis Receives EU Approval for Luxturna
The severity of chronic diseases has led to the demand for efficient medicines. The ability of regenerative medicine to treat severe life-threatening diseases in an efficient manner has created a huge demand for the products across the world. Increasing drug approvals have contributed to the rising uptake for Regenerative Medicine Market. In 2018, Novartis received usage approval from the European Union for its latest regenerative medicine ‘Luxturna’.
The drug was used to treat and restore sight for people with vision impairment. Luxturna was widely useful in treatment of rare retinal diseases. Fortune Business Insights states that the approval for Luxturna will contribute to the growth of the global regenerative medicine market in the forthcoming years.
Integra LifeSciences’ Latest Product Offering Will Favor Market Growth
The advancements in regenerative medicine have fueled their demand across the world. Increasing product launches have contributed to the rising uptake of regenerative medicine across the world. In 2017, Integra LifeSciences announced the launch of Integra Dermal Regeneration Template Single Layer ‘Thin’.
The product was aimed at repairing dermal defects in a one-step procedure. The medicine will also aid in reducing hospital stays. Fortune Business Insights has identified Integra’s latest product offering as a major Regenerative Medicine Market growth driver.
Get Sample PDF Brochure@ https://www.fortunebusinessinsights.com/enquiry/request-sample-pdf/regenerative-medicine-market-100970
Some of the leading companies that are operating in the Regenerative Medicine Market are Integra LifeSciences Corporation, CELGENE CORPORATION, Medtronic, American CryoStem Corporation, Tissue Regenix, Avita Medical, Osiris Therapeutics, Inc., Wright Medical Group N.V., Smith & Nephew, and Integra LifeSciences Corporation.
Research Methodology:
Fortune Business Insights follows a robust research methodology that involves data triangulation based on top-down, bottom-up approaches, and validation of the estimated market numbers through primary research. The information used to estimate the market size and forecast for various segments at the global, regional, and country level is derived from the most credible published sources and through interviews with the right stakeholders.
Growth rate or CAGR exhibited by a market for a certain forecast period is calculated on the basis of various factors and their level of impact on the market. These factors include market drivers, restraints, industry challenges, market and technological developments, market trends, etc.
SECONDARY DATA SOURCES THAT WE REFER TO:
Annual reports, investor presentation, SEC filings, and press releases of companies operating in the market
Studies published by relevant associations MedTech Europe; American College of Radiology; Cancer Council Australia; Japan Hospital Association, etc.), government sources (Centers for Disease Control & Prevention, Ministry of Health, Labour & Welfare, Japan; National Health Service, England, etc.), international organizations (World Health Organization, The Organization for Economic Co-operation and Development, Eurostat, etc.), and articles published by Research Gate, NCBI, etc.
Website, reports, and press releases of end user facilities – Hospitals, Ambulatory Surgery Centres, Clinics
Industry journals and paid databases
Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
https://www.reuters.com/brandfeatures/venture-capital/article?id=141445