Why is Psilocin Orally Active?
Why is Psilocin Orally Active?
Image: Kamiel Proost
Originally published: http://altdotmind.com/why-is-psilocin-orally-active/
This is the third article in a series on psychedelic chemistry, and the final article focusing on the tryptamine class. In the previous article we learned that though DMT and 5-MeO-DMT lack oral activity, chemistry wizards are able to change that. By making one of a variety of simple alterations to their structure they may be changed into analogs (“research chemicals”, or RCs), each possessing their own unique subset of characteristics including oral activity. That’s because the chemists changed the three-dimensional configuration of the molecules in such a way that the lone pair of electrons situated on the amine’s nitrogen (Figure 1) became shielded, thereby preventing their degradation by MAO. To recap, if one consumes monoamines (such as certain tryptamines) orally, MAO transforms them in the gut and by the time they enter the bloodstream they are no longer psychoactive – Figure 2.
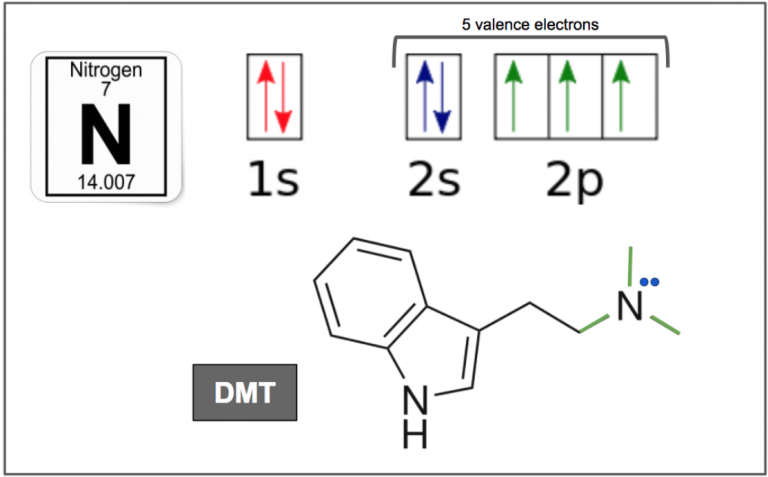
Figure 1. Nitrogen has 7 electrons in total, and 5 valence electrons. It has one electron in each of the three 2p orbitals, which allows it to make three bonds (green), and two electrons in the 2s orbital which exists as a lone electron pair (blue).
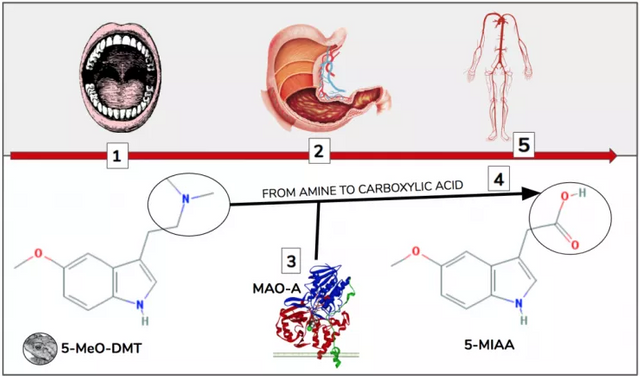
Figure 2. After 5-MeO-DMT is consumed orally (1) it enters the gut (2) and is transformed by MAO-A (3). MAO-A uses oxygen to convert the amine into a carboxylic acid (4). This converts 5-MeO-DMT into the nonpsychoactive 5-MIAA (5-methoxyindole-3-acetic acid), the species which enters the circulatory system (5)
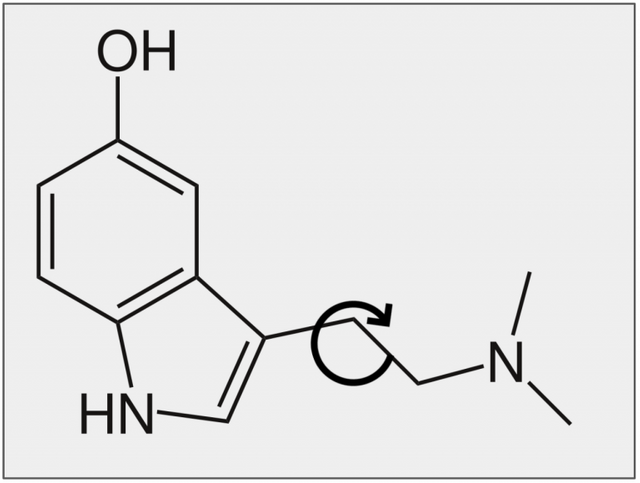
This article is going to unpack a study (Figure 3) that showed, by comparing the structures of the naturally-occurring molecules psilocin and bufotenin why the former is orally active while the latter is not. This is another pioneering study from the lab of Dr. David Nichols, who is, along with Albert Hoffman and Sasha Shulgin, in my estimation one of the three true giants of psychedelic chemistry. Its his work and excellent lectures from ESPD50, Psychedelic Science (2013 and 2017), and Breaking Convention that restoked my appreciation for chemistry and inspired me to not only deepened my knowledge, but also to start this series of articles. The outpourings from his majestic mind has fundamentally shaped the topics and content of these articles… Shout out Big D, whut-whut!
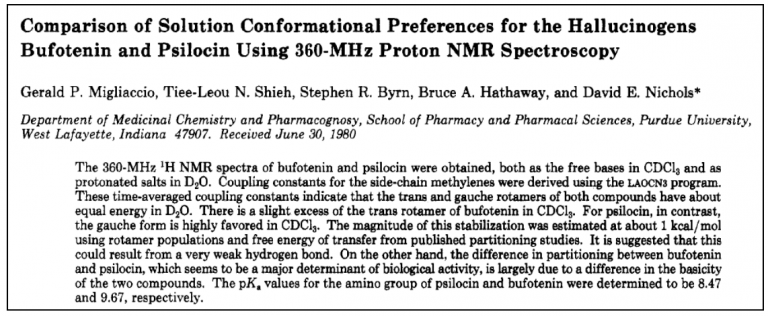
Figure 3.
The structure and atomic composition of a chemical are obviously critical to our understanding, and the progression of, chemistry and pharmacology. The problem with that is that molecules are small – really small. Even with today’s stupefying repertoire of advanced scientific analytical instruments, there is still no practical way for us to observe their structure directly. So instead we have devised sophisticated methods in which to do so indirectly. One of these methods is called Nuclear Magnetic Resonance (NMR) Spectroscopy, which uses information about the spin of atomic nuclei to determine what a compound’s structure looks like.
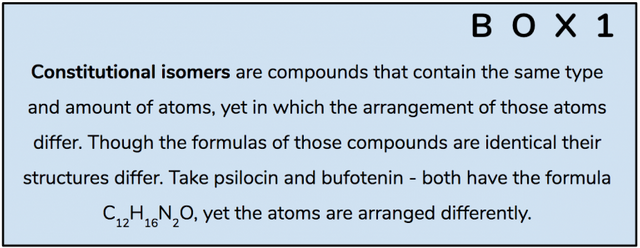
In 1980 the team at Purdue University used NMR spectroscopy to investigate how the three-dimensional structures of bufotenin and psilocybin differ from one another. Even though these two compounds are constitutional isomers (Box 1; Figure 4), there is a critical difference in their activity – psilocin is orally active, whereas bufotenin is not. This tiny change, moving the hydroxyl group from position 5 to 4 made this critical difference in the way they are absorbed by a human body. Though 2D-representations of the respective molecules are too low resolution to allude to the reason for the disparity, the researchers (correctly) suspected that by looking at their 3D-structures they would be able to understand why one molecule could resist deamination by MAO, while the other could not.
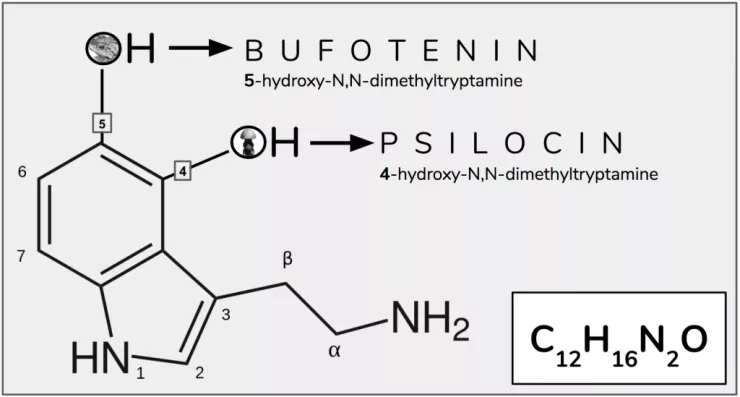
Figure 4. Bufotenin and psilocin are constitutional isomers, the only difference in their structure is the position of the hydroxyl group (-OH).
NMR spectroscopy revealed that the ethyl sidechain of bufotenin is able to rotate freely, meaning it can spin around on its own axis (Figure 5). That is however not the case for psilocin, something locks it in place, preventing it from rotating freely. The ethyl sidechains of the molecules are identical, which means that whatever is preventing the free rotation of psilocin’s ethyl sidechain is related to the hydroxyl group being situated at position 4, and not 5. To find out exactly what that was, the researchers used specialized software called LAOCN3. Before we explore what they found it would be useful to our interpretation of the results if we brushed up on a couple of elementary concepts in chemistry.
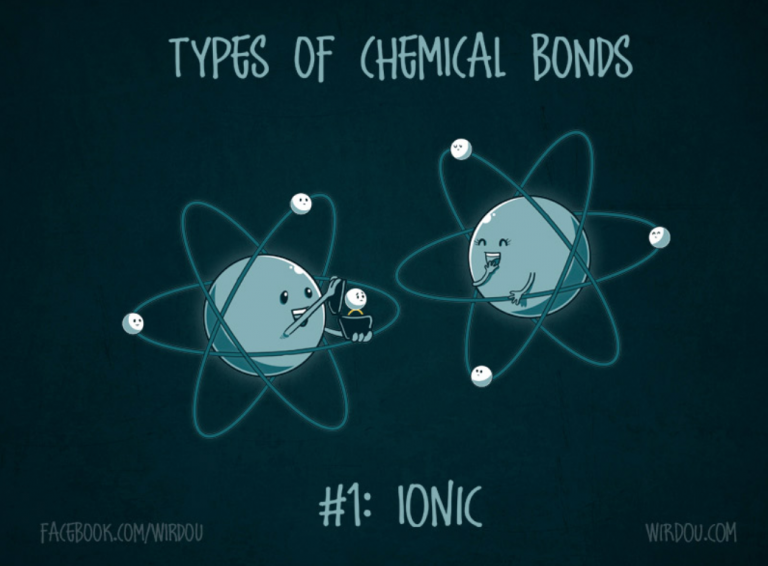
There are two basic types of bonds that atoms can form with one another. The first, called an ionic bond, forms when atoms exchange electrons with one another. This happens if the encountering atoms possess large differences in their respective affinities for electrons (called electronegativity), one atom really wants to lose an electron, while the other really wants to gain it (Figure 6). So an electron (or electrons) are exchanged, and because it is negatively charged the transfer changes the charge of each atom. The atom that gains the electron gains a negative charge and thus becomes negative, while the atom that loses the electron loses a negative charge and thus becomes positive. And as the old adage goes, opposites attract – the oppositely-charged atoms come together and form a stable bond with one another.
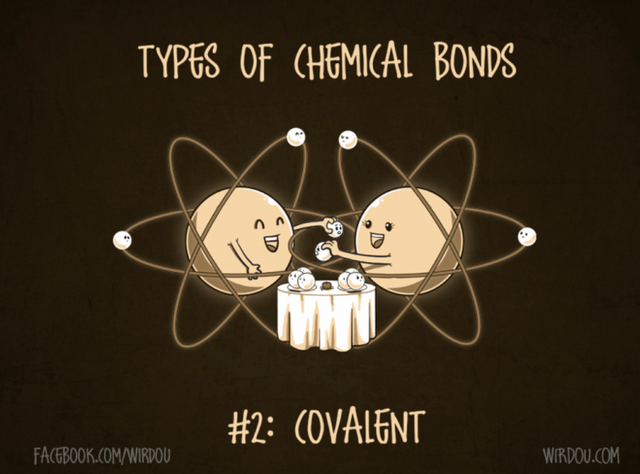
The other type of bond that can unite atoms is a covalent bond. This happens when atoms with similar affinity for electrons encounter one another, neither really wants to lose/gain an electron so they reach a compromise – they share their electrons among each other. Both atoms pretend that the electron that it shares, as well as the electron shared by the other atom, belongs to it (Figure 7). It’s this overlap of shared electrons that connects the atoms together into a single molecule.
Because there are no electrons that are transferred in the covalent bond the atoms don’t assume a charge as was the case with ionic bonds. However, that’s only partially true… In certain cases, the atoms that take part in a covalent bond do have some difference in their affinity – not enough for them to exchange electrons and form an ionic bond, but enough so that when they form a covalent bond and share electrons those shared electrons are closer to one atom than the other. This is known as a polar covalent bond. The atom to which the shared electrons are in closer proximity has a higher electronegativity and thus becomes partially negative (δ-). Conversely, the atoms with lower electronegativity are further from the shared electrons and are partially positive (δ+). Because of this asymmetrical charge, polar molecules are able to form weak bonds with other polar molecules, or with compounds that have a net charge. Now that we’ve covered some basic concepts let’s get back to the results of the study and apply what we’ve learned by taking a closer look at psilocin (Figure 8).
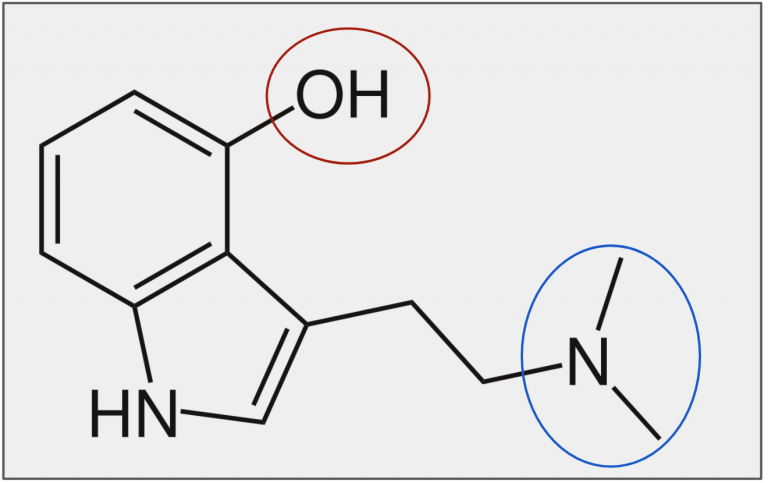
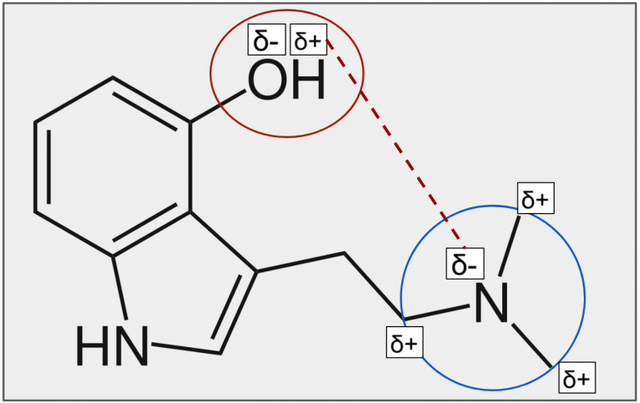
Figure 8. In the red area is a hydroxyl group (Figure 9), and in the blue area is a tertiary amine (Figure 10).
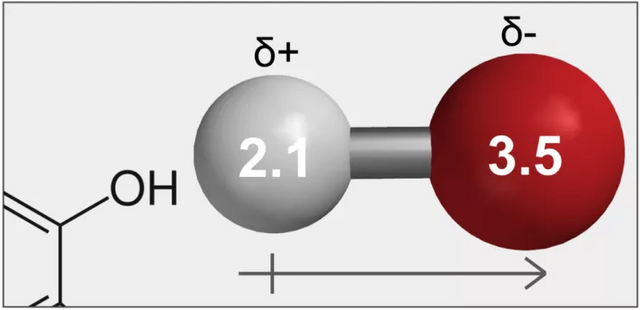
Figure 9. The electronegativity of hydrogen (white) is 2.1, while that of the oxygen (red) is 3.5. This difference of 1.4 in their electronegativity is not enough to form an ionic bond, but does lead to partial charges – oxygen has a higher affinity for electrons meaning the electrons are closer to it and assumes a partially negative charge (δ-), while hydrogen assumes a partially positive charge (δ+).
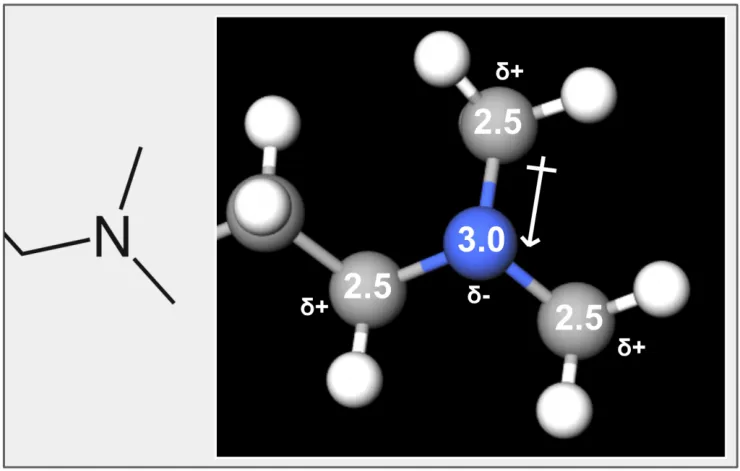
Figure 10. The tertiary amine group consists of a nitrogen (blue) with an electronegativity of 3.0, connected to three carbons (grey) each with an electronegativity of 2.5. Nitrogen has a higher affinity for electrons and pulls the electrons closer to it, leading to a partial negative charge (δ-), while the carbons have partial positive charges (δ+).
Taken together: psilocin has hydroxyl group at position 4 with a partially negative oxygen and a partially positive hydrogen, and an amine with a nitrogen that is partially negative and carbons that are partially positive. Because of these partial charges something interesting happens – the partially positive hydrogen from the hydroxyl group and the partially negative nitrogen from the amine attract one another (Figure 11).
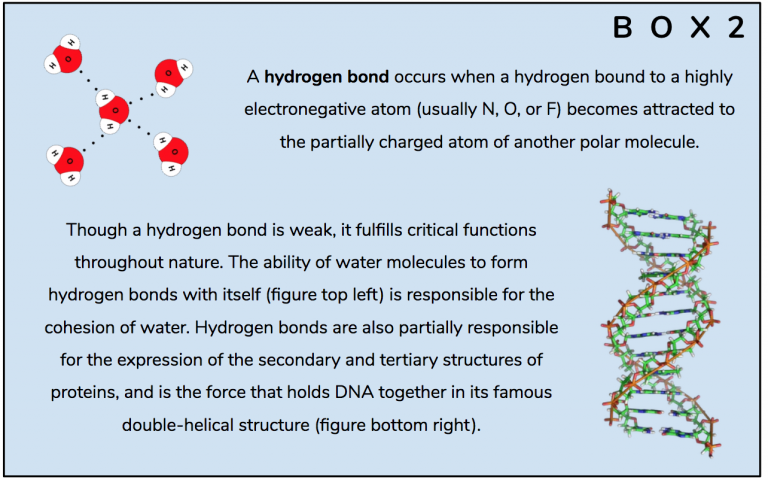
The hydrogen and nitrogen form a special type of bond with one another known as hydrogen bond (Box 2) which pulls the two atoms closer to one another, changing the shape of the molecule – Figures 12 and 13.
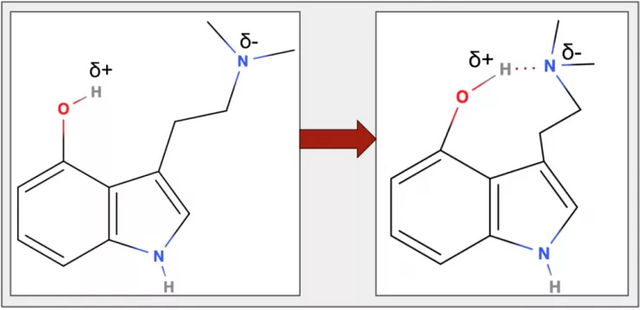
Figure 12. The partial positive charge on the hydrogen and partial positive charge on the nitrogen (left) are attracted to one another and form a hydrogen bond which pulls the atoms closer to each other, changing the molecule’s shape (right).
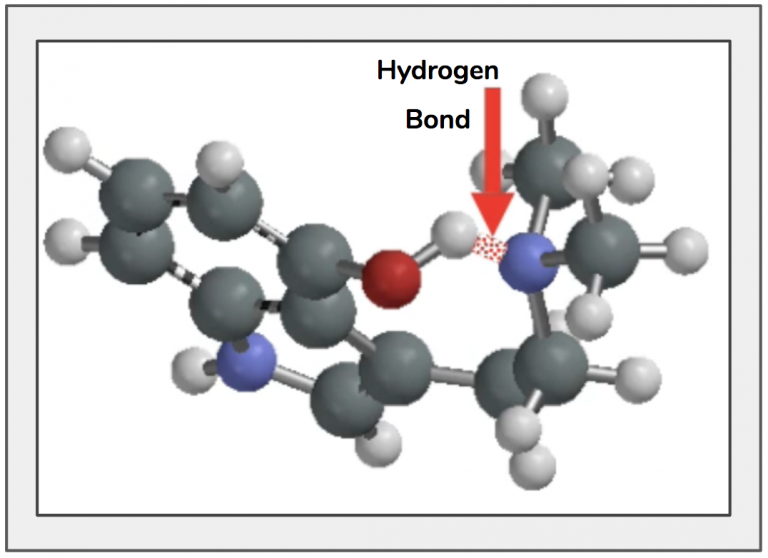
Figure 13. The hydrogen of the hydroxyl-group is bent backwards into a gauche conformation while the ethyl tail bends towards the indole ring to further shorten the distance between them.
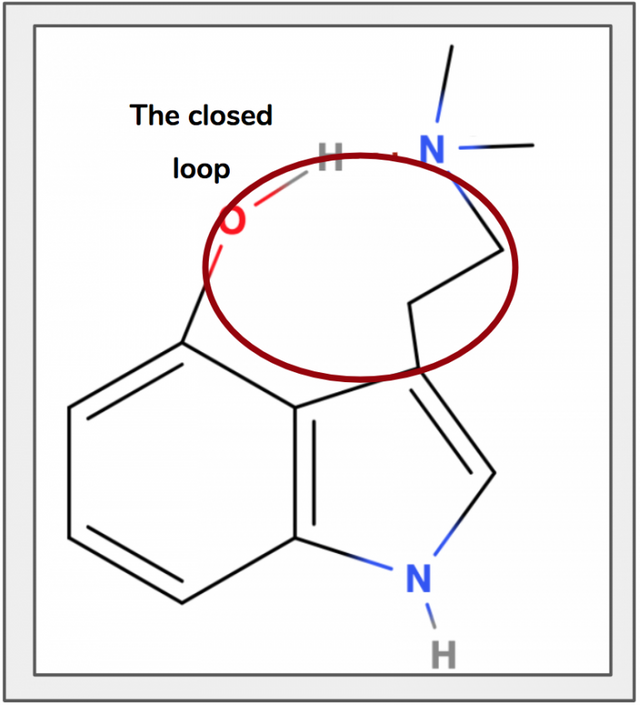
It’s this hydrogen bond that locks the ethyl sidechain into place by forming a closed loop (Figure 14), preventing it from rotating freely. In bufotenin the ethyl sidechain can rotate freely because no such hydrogen bond exists. Because the hydroxyl-group is at position 5 and not 4, the partially charged molecules are too far away from one another to form the hydrogen bond, change the shape of the molecule, and lock the ethyl sidechain into place.
But what has any of this to do with the difference in oral activity between the two molecules? Turns out, everything. It’s this hydrogen bond and closed loop formation in psilocin which shields the lone pair of electrons situated on the nitrogen. Because MAO cannot access the electrons it cannot deaminate the molecule – this is why it can pass through the gastrointestinal system unchanged.
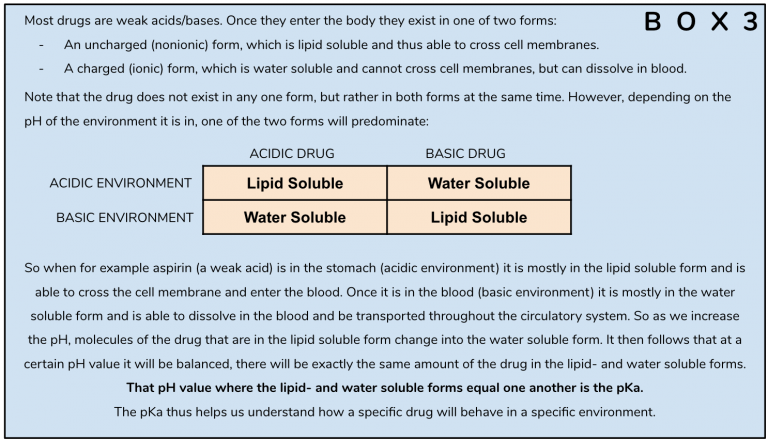
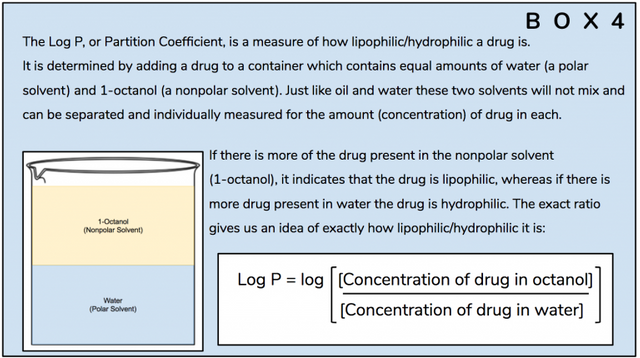
But there’s more. The hydrogen bond and resulting closed loop formation also lead to several other important changes in the property of the molecule which further accentuates its efficacy and potency as an orally-active psychedelic tryptamine. After generating 3D-models of the respective molecules, the researchers went on to compare their pKa (Box 3) and Log P (Box 4) values.
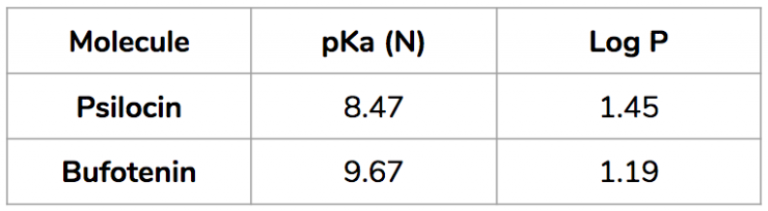
When they measured the pKa and the Log P for both psilocin and bufotenin they found the following:
The pKa for Bufotenin is 9.67, meaning that at that specific pH-value equal amounts of the molecule will be present in both the ionized (water soluble) and protonated forms (lipid soluble). When the molecule is in the blood, which has a pH of about 7.4, almost all of it (99.5%) is in the ionized form. In contrast, psilocin has a pKa of 8.47, closer to the pH of blood. So for psilocin, only about 52% is in the ionized form. That means that in the blood, 48% of psilocin will be in its unionized form versus only about 0.5% when it comes to bufotenin. As it is only the unionized form of the drug that can cross cell-membranes, this has profound implications for the potency of these two drugs – psilocin is not only able to better withstand degradation by MAO, but once it is in the blood there is also much more of it available in a form that can cross cellular membranes and thus can reach the target receptors and exert an effect.
The difference in pKa is also related to the shielding of the electron lone pair by the hydrogen bond. As we have learned, amines possess a nitrogen with a lone pair of electrons. These free electrons, which carry a negative charge, are all too happy to snap up positively-charged protons (H+) from a solution they are in. This is, according to the Bronsted-Lowry acid-base theory, the very definition of a base – something that accepts protons. When it comes to psilocin the lone pair of electrons are shielded and are thus much less likely to accept protons. As a consequence, psilocin is less basic that is bufotenin.
The researchers also detected a difference in the Log P values – 1.19 for bufotenin, and 1.45 for psilocin. In the Log P scale a negative value indicates a compound which is hydrophilic, whereas a positive value indicates one that is lipophilic. Both these compounds are thus lipophilic, and psilocin, with the higher value, is more lipophilic. For drugs, in general, it is preferable for them to be lipophilic so as to be able to cross cell membranes, but not too lipophilic because then they immediately migrate to, and are stored in, the body fat. Research indicates that a Log P value of about 3.0 is the “sweet spot”, so psilocin is closer to this number, again indicating that its properties are more favourable once it enters the body.
The researchers started with a simple question: how is it that two isomeric compounds with such a small difference have such widely different properties when they are consumed orally? With NMR Spectroscopy we learned that it all has to do with the fact that because the hydroxyl group of psilocin is a little bit closer to the amine it was able to form a hydrogen bond between the two groups. This hydrogen bond shields the electron lone pair from deamination by MAO, which means that, unlike bufotenin, psilocin is orally active. The hydrogen bond also decreases the molecule’s proton-accepting capacity thereby decreasing its pKa value which means that at blood pH there is more of psilocin in the non-ionized (lipid soluble) form which is able to cross cell membranes and thus enter the central nervous system (CNS). Finally, we saw that it also affected the Log P value, and that psilocin is a more lipophilic compound, closer to an ideal value for drugs to effectively enter and bind to the appropriate receptors in the CNS.
I hope you enjoyed this journey, in the next article we will start our exploration of the phenethylamine class.
About the Author
Faan Rossouw was born and raised in Cape Town (South Africa) and currently resides in Montreal (Canada). He holds a MSc in Plant Science, and is the co-founder and Chief Strategy Officer of Indeeva Biomedical, a medical cannabis company that focuses on producing condition-specific cannabinoid therapeutics.


Hi! I am a robot. I just upvoted you! I found similar content that readers might be interested in:
http://www.psychedelicstoday.com/2018/01/15/psilocin-orally-active/
What a fascinating article. I am definitely a science nerd but I am typically at a much larger scale, in rehabilitation, but with your refresher on the bonds and the graphics to support, I was able to follow and I learned quite a bit today about the chemicals that I have come to resepect deeply.
I was recently hearing about the popular fly agaric, with its muscimol, being ingested in two manners: oral ingestion and combusted within its own cap. The one who ate it reported feeling "drunk" which makes sense with it's depressive and sedative actions on the body. This article made me curious about if one of those methods has a large advantage over another, as far as availability to the receptors?
Thank you! Yes, Faan has done a great job with this series.
As for the fly agaric, I recently just watched one of the new episodes of Hamilton's Pharmacopeia, and the people who he was hanging out with both drank and smoked the mushroom. It makes me wonder if smoking breaks down some of the not-so-pleasant compounds in the Amanita muscaria. As for psilocybin, I have heard about some folks smoking it, but I am not entirely sure how effective that is. Maybe if it was smoked with an MAOI. Creating a tea makes the effects come on quicker and more intense since it does not need all the time to be broken down.
Great article, I will be sharing this to my FB page.
Thank you! :)