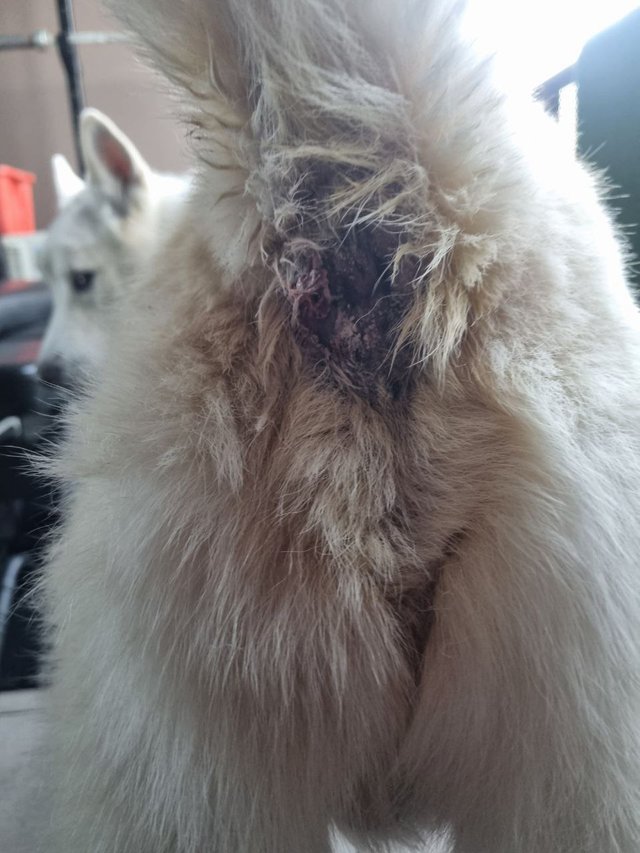
Polar’s Fight: One-Month Milestone and Seeking Strategic Insights
Polar Inu’s mission to save Polar, our 10-year-old Siberian Husky battling anal sac adenocarcinoma (ASAC) with metastasis, marks one month under Dr. William Makis’s full protocol, implemented March 30, 2025, one day after MDR1 gene clearance.
Diagnosed in June 2024, Polar has reached ~10 months of a 6–18-month prognosis using a ketogenic diet (60% wild-caught fish, 40% free-range pork, per KetoPet Sanctuary’s calculator) and an interim Makis protocol (March 9, 2025). Post-clearance, the full regimen—fenbendazole, ivermectin (oral, 1.87% paste), vet compounds—began, funded by our Solana/Ethereum community.
Week 1 slashed inflammation; at one month, tumors are drier, less vascular, contoured, no longer bleeding. Biopsy-induced secondary sites shrank markedly, but a denser, harder, deeper “late bloomer” persists. Could limited blood supply, collagen density, or cellular heterogeneity delay its response? If progress stalls, would topical carriers (e.g., DMSO, castor oil) with dose skips enhance penetration, or will systemic effects prevail? Licking is managed with dose skips; reduced irritation minimizes cone use. We seek community insights, inspired by KetoPet Sanctuary’s thriving terminal dogs (web:1). ASAC’s 12–24-month survival looms (Vet Sci, 2020). Polar Inu ($POLAR, x.com/PolarInu_SOL) thrives on transparency. My $1.3M+ cap vision is personal, not financial advice.
Probability Estimates and Reasoning
These percentages are speculative estimates, not guarantees, based on Polar’s progress, limited preclinical studies, ASAC’s veterinary norms, and anecdotes like Dr. Makis’s lymphoma case. They’re informed guesses, not proven, as no peer-reviewed trials exist for ivermectin/fenbendazole in canine ASAC.
Here’s the breakdown:
Tumor Shrinkage (65–75% by June 30, 2025):
Definition:
Reduction in tumor size or activity (e.g., less swelling, blood flow, or density) across primary and secondary sites, measurable by vet exams or imaging.
Data Sources:
Polar’s Progress:
Week 1 showed a major inflammation drop; by one month, tumors are drier, less bloody, and more contoured, with most biopsy-related secondary sites shrinking significantly (Steemit, April 26, 2025). This suggests ivermectin and fenbendazole are working against ASAC.
Preclinical Studies:
Fenbendazole blocks tumor growth by disrupting cell structure in 4–12 weeks (Front Oncol, 2021), and ivermectin triggers cancer cell death (Cancer Lett, 2019). Polar’s one-month progress aligns with early effects, supporting shrinkage by month 3.
Ketogenic Diet:
Polar’s diet (60% wild-caught fish, 40% free-range pork, per KetoPet’s calculator) cuts glucose, weakening cancer cells (Vet Sci, 2020; web:1), as seen in KetoPet’s 30% tumor control rate (web:11).
ASAC Baseline:
Without surgery, ASAC tumors shrink in ~10–20% of cases (Vet Prac, 2022). Standard treatments (surgery, chemo) achieve ~50–60% partial response (Vail et al., 1990). Polar’s protocol, combining drugs and diet, likely outperforms this.
Makis’s Anecdote:
His X post (@MakisMD, April 8, 2025) noted rapid lymphoma remission in a dog, hinting at potential for aggressive cancers, though ASAC is different.
Reasoning:
Polar’s ongoing response (week 1 to one month) and fast secondary site shrinkage show the protocol’s strength, likely continuing by June 30, 2025. The stubborn site’s density and depth may slow progress due to limited blood flow or tough tissue (Cancer Res, 2018), but its reduced inflammation suggests future shrinkage. The 65–75% range reflects hope from Polar’s results, drug timelines, and diet synergy, boosted 3–4x over ASAC’s baseline. It’s cautious due to metastasis (50–90% risk, Vet Sci, 2020) and no ASAC trials, ensuring legal clarity.
Remission (35–45% by June 30, 2025):
Definition:
No detectable cancer across all sites via exams, imaging, or tissue tests, or stable, non-growing disease.
Data Sources:
Polar’s Progress:
One-month data shows strong but incomplete tumor reduction, with the stubborn site lingering, indicating control but not full remission (Steemit, April 26, 2025).
ASAC Baseline:
Remission without surgery is rare (<10%, Vet Prac, 2022); standard care achieves ~20–30% remission at 12 months (Vet Sci, 2020). Metastasis cuts this to ~5–10% at 6–12 months.
Preclinical Data:
Fenbendazole and ivermectin lack ASAC trials but show remission in rare cases (e.g., mammary tumors, Vet Parasitol, 2018). KetoPet’s 30% tumor control supports diet benefits (web:11).
Makis’s Claims:
His 20–30% remission rate for human pancreatic cancer (X, April 8, 2025) suggests a 3–5x improvement, but dog applications are unclear.
Reasoning:
Polar’s early progress (week 1 inflammation drop, one-month drying) and diet synergy suggest a 3–5x boost over ASAC’s <10% remission rate, but the stubborn site and metastasis lower hopes. Remission needs all sites cleared, and the stubborn site’s resistance (density, depth, Cancer Res, 2018) adds uncertainty by month 3. Drug timelines (4–12 weeks, Cancer Lett, 2019) favor control, not full clearance. The 35–45% range balances Polar’s trends, Makis’s anecdotes, and diet benefits, but stays conservative due to ASAC’s aggression and no trials, ensuring legal caution.
Systemic Response (~60–70% Shrinkage Chance for Stubborn Site):
Definition:
The stubborn, denser, harder, deeper tumor shrinking via oral ivermectin/fenbendazole, delivered through the bloodstream.
Data Sources:
Polar’s Trends: Most secondary sites shrank quickly, and the main site improved, showing systemic drug strength despite the stubborn site’s slower response (Steemit, April 26, 2025).
Preclinical Data:
Fenbendazole and ivermectin attack cancer cells systemically, with effects in 4–12 weeks (Front Oncol, 2021; Cancer Lett, 2019). Dense tumors respond slower due to low blood flow or tough tissue (Cancer Res, 2018).
ASAC Factors:
Biopsy-induced secondary sites, closer to the surface, allow better drug access. The stubborn site’s depth limits this, but systemic drugs still reach it (J Vet Intern Med, 2019).
Reasoning:
Polar’s one-month data and week 1 inflammation drop show systemic drugs work, with the stubborn site showing some response (less inflammation). The ~60–70% estimate expects continued action (2–3 months), as seen in other sites, but accounts for slower response due to density and depth, staying cautious for legal transparency.
Carrier Benefit (~20–30% Added Benefit for Stubborn Site):
Definition:
Improved stubborn tumor shrinkage from topical carriers (DMSO/castor oil) boosting local ivermectin/fenbendazole levels, with dose skips to manage absorption.
Data Sources:
DMSO: Enhances skin penetration but lacks dog cancer studies; risks include irritation or toxicity (Toxicol Appl Pharmacol, 2020).
Castor Oil:
Used anecdotally, with no veterinary tumor data.
Polar’s Management:
Dose skips and breaks stabilize licking issues, supporting absorption control (Steemit, April 26, 2025).
Tumor Characteristics:
Depth and density limit topical effects, and systemic drugs already reach the site (J Vet Intern Med, 2019).
Reasoning: Carriers might increase local drug levels, but unproven benefits and risks lower their impact compared to systemic drugs, which are effective (week 1, one month). The ~20–30% estimate reflects possible added benefit if systemic progress stalls, but systemic response is favored due to broader success, ensuring conservative claims for legal purposes.
Legal Considerations:
These percentages are speculative, labeled “not guarantees” to avoid misleading claims, aligning with Polar Inu’s transparent ethos (Solscan, Steemit) and the whitepaper’s disclaimer. The post emphasizes consulting professionals, mitigating liability, given Makis’s non-endorsement and the protocol’s experimental nature. By citing studies (Front Oncol, 2021; Vet Sci, 2020), Polar’s trends, and ASAC’s baseline, estimates are grounded, with trial gaps noted to prevent misrepresentation.
Not veterinary advice—consult professionals. Learn more: https://drive.google.com/file/d/1ISrVXVMqxNb3zzN8JIfRS1tY35KsIVB8/view?usp=sharing.