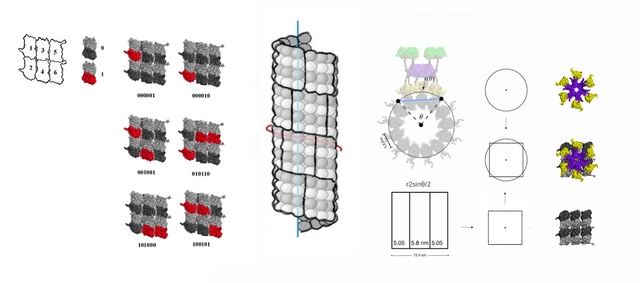
Why a 6-tubulin memory byte?
CAMKII has six arms, with one kinase domain each (double that actually, one on top and one on bottom), and is equipped to switch the state of up to 6 tubulin at once. Craddock, Tuszynski and Hameroff suggested a 7-tubulin memory byte with a central "address dimer"[1], but an addressing system could be external, and made up of microtubule-associated proteins (MAPS) on top of the microtubule (i.e. not encoded in tubulin dimers).
The model with 6-tubulin memory bytes also predict that neurons would have B-lattice type MTs, and that was what Kikkawa and Ishikawa described in 1994.[2] They also describe a "seam" and a 13-3 MT type, but it is possible that it could be an artefact from freezing the MTs, causing their shape to be shifted with 1 monomer forming a discontinuous lattice, while in vivo, they are 13-4 and continuous.
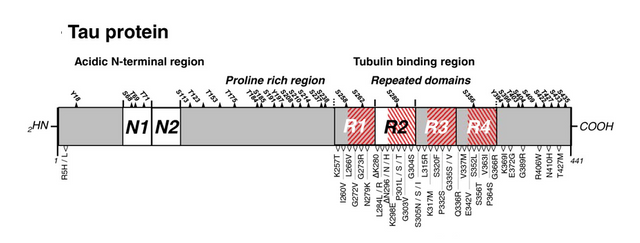
For the addressing system, tau proteins could be a candidate. Tau proteins are associated with Alzheimers and memory loss, have been suggested to be involved in addressing for motor proteins, and overall look a bit like a barcode. When the addressing system is moved from a central "address dimer" to an external layer, as in one that is external also to tubulin dimers, a form of structure on top of the microtubule built entirely with MAPS, then there would be higher resource efficiency with 6-tubulin bytes simply because every single tubulin can be used as a molecular switch for storing memory.
What needs to be researched?
6-tubulin memory blocks need to be able to fit on the type of microtubule that they form, a 13–4 B-lattice perhaps. The coil angle needs to be 4 monomers, what is often called a 4-start type MT, unless the number of filaments is divisible by 3, 15 proto-filaments (PF) for example.
13–4 B-lattice type microtubules are not widely described, but I'm not sure how detailed the data that exists is, are the microtubules that are measured complete with MAPS, as in, the way they exist in the cell, if MTs and MAPS are seen as a single integrated system, or, are they isolated in some way, perhaps lacking some MAPS that would influence the shape in vivo?
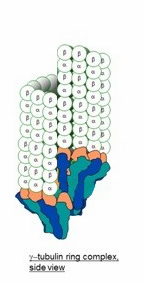
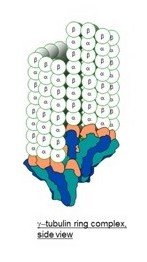
The microtubule root complex as a possible site for proof of a 4-start helix
The model for a 6-tubulin memory block predicts that the microtubule root complex would look like this, a 4-start repeat would be reflected there.
The root complex is often depicted like this, a 3-start, but that could be a priori, from that a 13-3 structure is an artefact of freezing the cell before photography. Memes like "they are discontinuous" can survive and propagate even if they are wrong, Jung's global unconscious was about that, how memes in the meme-pool of society propagate not through reasoned examination but more sloppy.
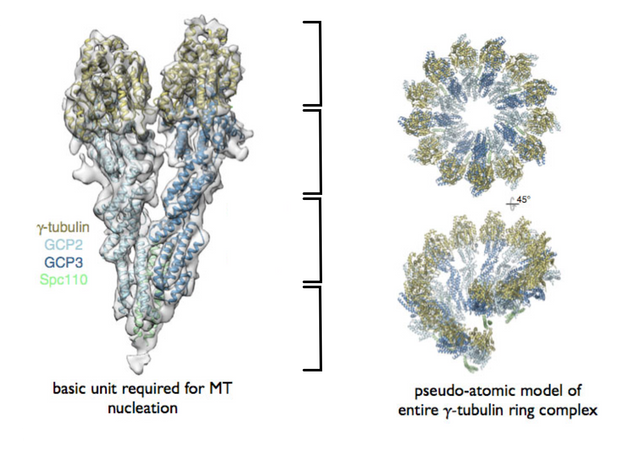
The γ-tubulin small complex (γTuSC) is two tubulin monomers wide at the top, and forms a one-turn coil, 7 γTuSC in total with an overlap of 1/2 γTuSC .
The model for 6-tubulin memory blocks predicts that the γTuSC occupies around 16 nm in height, rather than the 12 nm of a 3-start MT, or that it together with other root complex proteins forms a 16 nm high structure, so that the one-turn coil is the same height as 2 tubulin dimers, and supports a continuous 13-4 helical B-lattice type MT with 6-tubulin memory blocks.
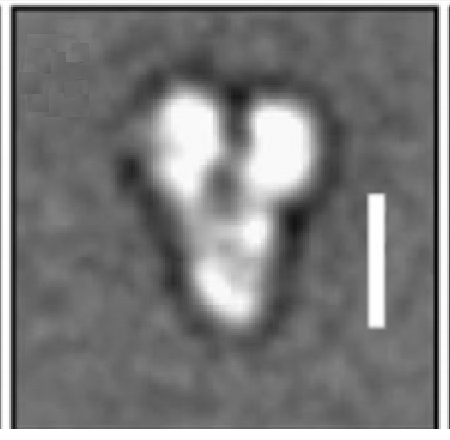
The image below is from a paper that included a 10 nm reference bar[3], it looks closer to 16 nm (two tubulin dimers) than 12 nm (1.5 dimers. )
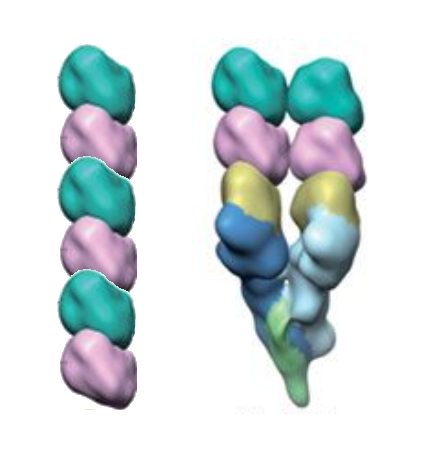
and another paper[4] shows proportions that seem to support a 4-start helix (I have added the reference two dimer column on the left. )
interesting details you shared quite an in depth post to know more about it