⚡ Home School: Science - Apple Powered Clock
I'm back again with some more home school science - this time, an Apple Powered Clock (🍎⚡🕰️)
I got such a nice response to my last home school post (Skittles Diffusion) and since the-6yo-gorilla has picked up Chicken Pox from the-3yo-gorilla, it was the perfect opportunity to do some more Home School Science.
I love that my eldest enjoys home schooling (he says that I'm his favourite teacher (🦚) so when I asked him what he wanted to do today, he picked up a pen, got a piece of paper and top of the list - Science.
I picked up a "Kitchen Lab" science kit from a charity shop a while back and having already done an "freezing ice cube experiment" (water won, sugar water & Orange Juice in joint second, salt water last) and the "fishing ice cubes" (which didn't work), next up was the Apple Clock.
It's important to point out that this isn't a clock made by Apple. This Apple clock will clearly have a far superior battery life.
What you need
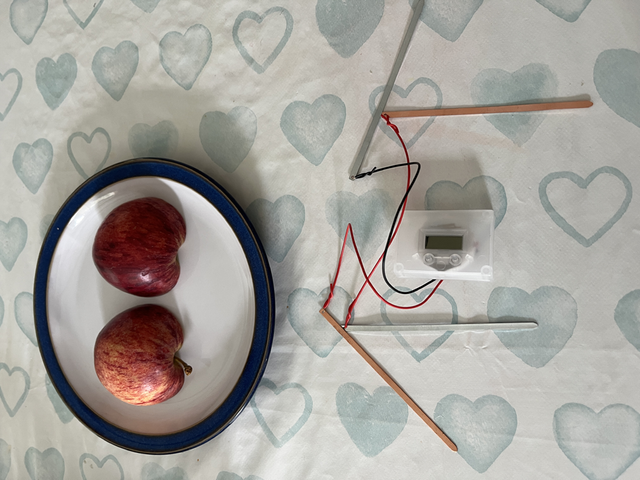
The main components came with the kit -
- The clock (with wires)
- 2 x brass electrodes
- 2 x zinc electrodes
- Additional wire
And I needed to add -
- A plate (or other apple holding mechanism)
- An apple (or alternative juicy fruit)
- A knife (for cutting the apple in half)
Method
Setting it up was simple. The wires from the clock connect to one of the brass and zinc electrodes and then the additional wire connects to the other brass and zinc electrode.
You then complete a circuit by putting one clock connector into each half of the apple, and the opposite from the additional wire into each piece of apple (i.e. the brass from the clock goes with the zinc from the additional wire).
And that's it! All you need to do after that, is set the time!
7 hours later - and the clock's still working well. If only all products had such a reliable battery.
How does it work?
I'll let Professor Molly Cool explain:
A chemical reaction takes place inside the apple to complete the circuit. The brass electrodes dissolve in the apple juice and release electrons. These move through the juice to the zinc electrode and forms an electrical current.
The electricity travels between the brass and zinc electrodes, then into the clock to power it before going back to the first brass electrode again. This repeats until the brass electrode loses too many electrons and stops working.
I should probably get an Ammeter to see if this could power a light in the event of a power cut 🤔
Please excuse the current state of my comments and replies, I'm currently downvoting various scammers and they don't like it.








Cool! I was always wondering how this works; Now i know ;)
Yeah, would be interesting if a lamp would work with that technique, or perhaps with an bigger apple, or by adding something else🤔 many ways to experiment with ;)
🙂 I don't think a bigger apple would help - I suspect that if I put the electrodes closer together, there'd be less resistance but I won't pretend to be a scientist!
The internet will probably tell me what kind of current's being produced - oftentimes, a battery that's too weak for a torch can run a clock for a long time so my guess is that it wouldn't work.
Your little one will be an engineer when he grows up, right...?
By the way, what kind of stubborn mate have you got there? He is... You must be a very bad person ;-))
Have I mentioned that before or did you intuit it from my writing?
Yeah - I've got a lot of haters at the moment 🤣
It was interesting to read you. It is often difficult to find people who think outside the box. Do the naysayers matter? Even though I knew about the topic, I liked the way you handled the whole text, it was enjoyable, it was great. It turned out to be a very good idea to spend time with my child.
Thanks and I think any time spent doing science with children has to be good for them.
This post/comment has been upvoted to compensate you for the downvote by bullionstackers on this post/comment.
For more detail and how to support this initiative, please read this post.
You can also follow our upvote trail on BottoSTEEM to help support other authors that have been targeted.
Note: The main compensating upvotes may take up to 2 days to arrive
This is so cool. How long did the battery last for the clock in the end?
😀👍👍👍