⚗️ Problem 1.10 – Chemistry by Chang (14th Edition) | Chapter 1: Measurement and the Properties of Matter
Greetings, Steemit learners! 👋
Here’s another quick and essential chemistry problem from Chapter 1 of Raymond Chang’s Chemistry (14th Edition) — a perfect warm-up for mastering density and unit conversion, foundational topics in any chemistry course.
🔹 Problem 1.10:
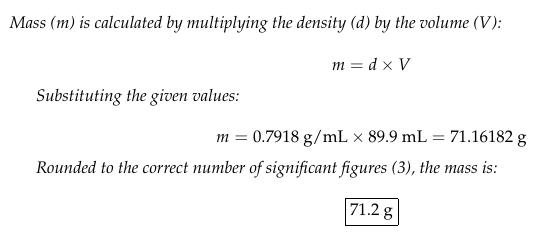
The density of methanol, a colorless organic liquid used as a solvent, is 0.7918 g/mL. Calculate the mass of 89.9 mL of the liquid.
Methanol is commonly used in labs and industry, and knowing how to compute its mass based on volume and density is a basic but important skill.
📸 Here is the full solution, step by step:
Are you currently studying Chemistry from Chang's textbook too? Let’s support each other — comment with your thoughts or approach!
Follow for more solved exercises, science content, and educational discussions. 🌟
#Chemistry #Chang #SteemitEducation #Density #Methanol #StudyWithMe #ScienceOnSteem