Saponification and Organic Handmade Soaps
Today in this post I will talk about part of my personal project, which consists of the production of organic soaps, which are commonly called or called handcrafted by the fact that in its preparation, does not intervene any industrialized machinery for its fabrication process.
But first we must know that it is a soap.
Soap is an agent or cleaning medium whose manufacture is based on the use of oils, vegetable fats and animal fats.
Chemically speaking, it is the sodium or potassium salt of a fatty acid that is formed from the reaction of oil, animal or vegetable fat with an alkali.
Now what is an alkali?
The alkaline colloquially is the union or mixture of soda or potash with water, it is noteworthy that this process is carried out once the amount of water necessary for the oil or oils destined for the preparation of our saponification has been calculated, and the The way to do it safely is to add soda or caustic soda preferably in 99% flakes to the water, since an exothermic reaction is produced which produces a lot of heat, it should be emphasized that this process should be carried out in a plastic container preferably or a stainless steel container, never in aluminum containers. It is important to use the appropriate personal protective equipment for this purpose, panty, nitrile gloves, safety glasses and anti-gas mask with special filters against chemical gases, in addition to having vinegar available in case any kind of splash occurs in the skin.
The origin of the soap is quite old, some say it was discovered in Italy after observing that the mixture of rainwater with the fat of the sacrifices of the animals that were made in the mountains and the resulting ashes product of the burning of the mentioned sacrificial rituals at the time, that material of ashes and fats was dragged towards the rivers and when arriving at this it formed an abundant foam which facilitated the cleaning of the dirty hands and the clothes that the women washed at the foot of the mountain Toad that is found specifically next to the river Tiber, from there etymologically comes the term saponification.
It is noteworthy that the ancient Egyptians and Greeks elaborated their own soapy products long ago based on water, oils and waxes, but historically it was the Romans who introduced their expansion, since they elaborated them by hand and used it as a means to avoid diseases on the skin for its cleaning power.
In the time of the middle ages it spread throughout Europe but it was only within the reach of the upper classes, due to its high cost for taxes that included in the elaboration of it, passing its period use, until reaching our days where the elaboration is basically industrialized.
Having said this brief account of the origin of soap, we must know what is saponification?
It is known as saponification to the chemical reaction that occurs when a fatty acid is mixed with an alkaline (alkaline) medium that I mentioned earlier, this means separates glycerin and fatty acids. So when mixing the oil with water next to the alkaline medium which is in this case soda or caustic soda (NaOH) or potash (KOH), soap is obtained and the byproduct which is glycerin, the latter at the industrial level is separated from the soap for its high sale value.
So summarizing
Soda (NaOH) or potash (KOH) + Water + Oil or fat = Soap
To proceed to perform the saponification, it is necessary to know how to calculate the amount of Sosa or potash necessary for the amount of oil and the type of oil that we use.
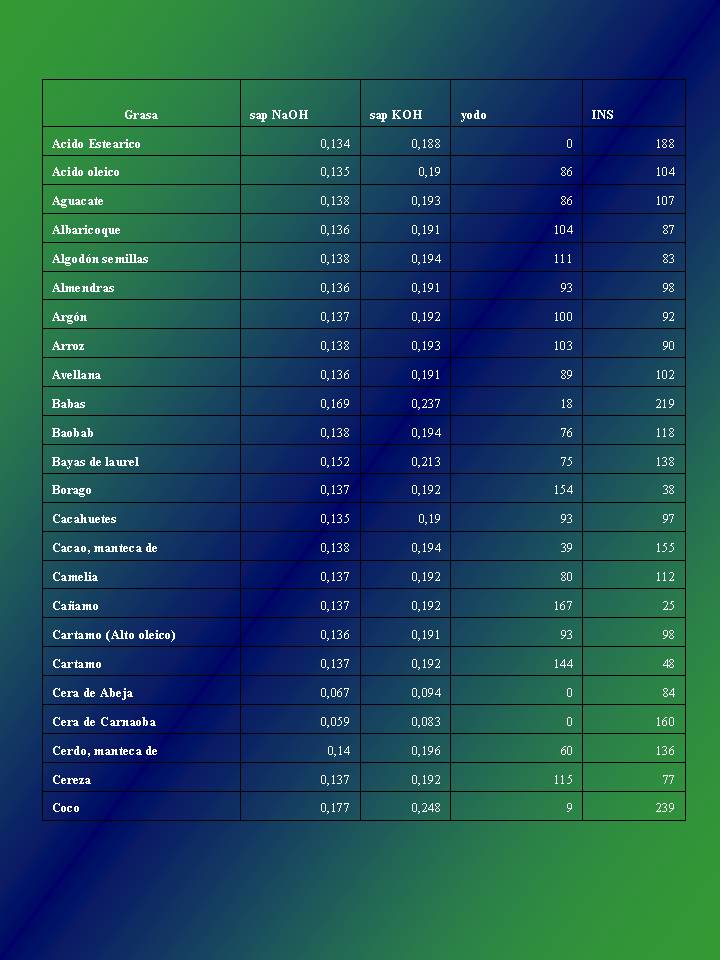
Here I show you the saponification table of the oils of the page medrulandia.
As you can see in the first column the type of oil appears, the second column Sap NaOH shows the alkali for solid soaps, the third column sap KOH shows the alkali for liquid soaps, the fourth column the value corresponding to the iodine that determines the tendency to rancidity and the fifth column shows the saturation index of the chosen oil.
Then to obtain the amount of soda needed to saponify an oil or fat, you must multiply the amount of oil or oil by the corresponding value that appears in the table described above.
For example if we have 1000 Grs of coconut oil (in the table its value is 0.177), the multiplication would result in the amount of soda or caustic soda necessary to perform the saponification of that amount of oil.
This is 1000 Gr of Coconut Oil * 0.177 = 177 Grs of Sosa
Now to calculate the water necessary to dilute the soda or caustic soda as it is commonly called, one third or third of the weight of the oils must be considered, that is, 33.3% of the total weight of the oil.
For the case of coconut oil
Then it would be: 1000 Gr of coconut oil * 33.3.% Or what is 0.33
1000 Gr coconut oil * 0.333 = 333 Grs of water
Now having everything clear, and taking into account that we already have the necessary alkali and oil to carry out the saponification process and having the personal protection tools such as safety glasses, organic anti-gas mask, nitrile gloves, apron, etc. .
We start by having our alkali in a plastic container and begin to pour our oil as if it were a thread while we beat with the help of a wooden pallet in a clockwise direction for about 25 minutes or until it forms what is called as a trace or mayonnaise point, just at that moment when you reach that point where you can make a line and notice that it does not fade, it is time to stop the shake and let it rest in the same container or pour it into the plastic containers that are destined for the soap.
After 4 days we proceed to demold the base soap protecting hands with nitrile gloves and placing this soap base in an airy place where sunlight does not hit for about 40 days, I personally prefer 50 days to be able to use the base soap directly or for the recasting process.
Which I will talk to you in the next post.





Hola @zubzero1974, upv0t3
Este es un servicio gratuito para nuevos usuarios de steemit, para apoyarlos y motivarlos a seguir generando contenido de valor para la comunidad.
<3 Este es un corazón, o un helado, tu eliges .
: )
N0. R4ND0M:
5309 7087 4295 2097
3551 9442 4866 4016
9405 2230 3096 3195
6281 1693 8422 9949
Congratulations @zubzero1974! You received a personal award!
Click here to view your Board
Congratulations @zubzero1974! You received a personal award!
You can view your badges on your Steem Board and compare to others on the Steem Ranking
Vote for @Steemitboard as a witness to get one more award and increased upvotes!