Gases // Kinetic Theory
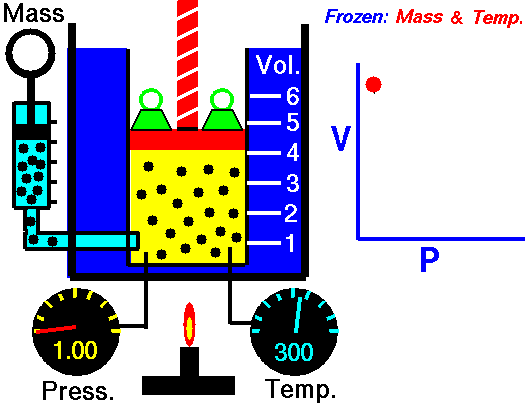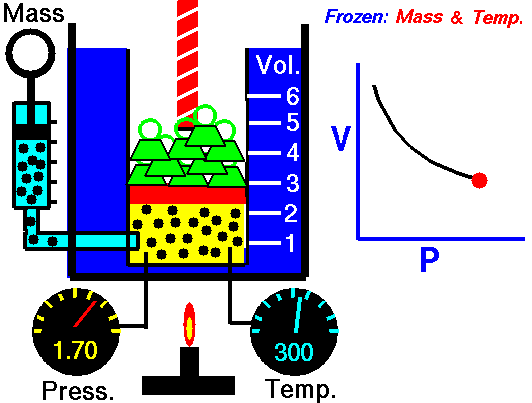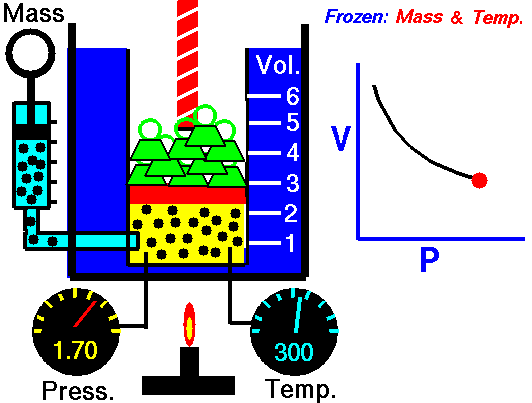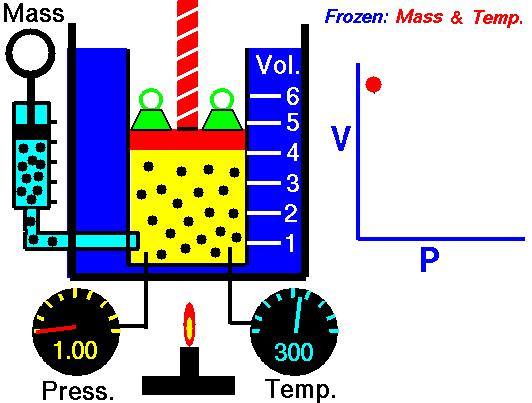
Animation: mass and constant temperature, Source of image of mastery of Wikimedia Commons

Robert Boyle (1627-1691), Source of image of mastery of Wikimedia Commons
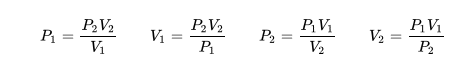

This law is a simplification of the law of the ideal or perfect gases distinguished for isothermal processes of a certain mass of constant gas, Source of image of mastery of Wikimedia Commons
The description of the behavior of the gases according to his pressure, volume and temperature obeys the call laws of the gases, which they confirm that said magnitudes they are related between if and that coincide with the results of the kinetic theory of the gases. Let's consider first the existing relation between the volume and the pressure of a gas, the above mentioned relation expresses itself by means of the law of Byle-Mariotte, which they affirm for a constant temperature, the pressure exercised by a gas mass on the walls of the receptacle, which contains it is inversely proportional to the volume, which the above mentioned gas occupies.
The law expresses as P.V = constant, for a certain temperature, where P is the pressure and the Vth represents the volume, from point of view of the kinetic theory of the gases, this law is correct, since, after diminishes the volume, which occupies a gas to constant temperature, the atoms or molecules, which compose it must collide with major frequency with the walls, from what it has to increase the pressure.
The relation between the volume and the temperature comes given by the first law of Gay-Lussac, who affirms that for a pressure, which should stay constant, a proportionality exists between the volume that occupies the gas and his temperature, where the relation is V/T = constantly, where the Vth is the volume and T is the absolute temperature, for this motive also this theory it is acceptable, since the increase of the temperature, it does that I increased the energy of the atoms or particles. The above mentioned increase must translate to itself in a major speed and shock more fortresses with the walls of the receptacle, since it is necessary that it increases the volume.
Bibliographical source
General physics - Page 268 for Santiago Burbano de Ercilla, Carlos Grace Muñoz - 2003.
General chemistry. Introduction to the Theoretical Chemistry - Page 257 for Cristóbal Valenzuela Calahorro - 1995.

