🧪 Chemistry by Chang – Problem 1.25 | Precision and Accuracy | Steemit Science
Hello Steemit learners! 👋📏
This exercise comes from Raymond Chang’s Chemistry (14th Edition), in Chapter 1: Measurement and the Properties of Matter. It focuses on two fundamental ideas in experimental science: precision and accuracy.
🔹 Problem 1.25:
Three students (A, B, and C) are asked to determine the volume of a sample of ethanol. Each student measures the volume three times with a graduated cylinder. The results in milliliters are:
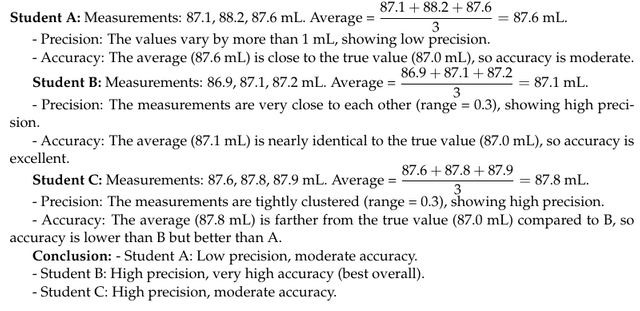
- A: (87.1, 88.2, 87.6)
- B: (86.9, 87.1, 87.2)
- C: (87.6, 87.8, 87.9)
The true volume is 87.0 mL.
👉 Comment on the precision and the accuracy of each student’s results.
This problem is excellent for reflecting on how measurements can be:
- Precise: values are close to each other (little variation).
- Accurate: values are close to the true value.
Understanding the difference between the two is critical in any laboratory setting.
📸 Here’s my complete analysis of each student’s results:
Which do you think matters more in the lab: precision or accuracy? Let’s discuss in the comments! 🧠💬
Follow me for more chemistry exercises, detailed breakdowns, and science practice from Chang’s Chemistry.